All published articles of this journal are available on ScienceDirect.
Dorsal Root Ganglion Stimulation for the Management of Chronic Neuropathic Pain: A Retrospective Case Series during Four Years follow-up in a Single Center
Abstract
Objectives:
The dorsal root ganglion (DRG) is involved in the transduction of pain signals to the central nervous system (CNS) and undergoes a number of physiopathological changes during chronic pain. The purpose of this data collection was to evaluate the long-term safety and efficacy of DRG stimulation for the treatment of chronic pain and its impact on functional aspects.
Materials and Methods:
Forty-four subjects with non-reactive chronic neuropathic pain syndrome were implanted with DRG stimulation.
Patients were evaluated at baseline as well as at 15, and 30 days, and at 3, 6, 12, 24, 36 and 48 months after medical intervention/surgery using the Visual Analogic Scale (VAS), which measures pain intensity, and the Oswestry Scale, for the estimation of disability (ODI).
Results:
After four years of simulation, VAS and ODI showed a statistically significant reduction throughout the follow-up period. The average pain relief obtained after 48 months of treatment was 74.1% ± 3.4.
Conclusion:
The results of this data collection demonstrate the feasibility of DRG stimulation, the correspondence between the clinical indications at the DRG implant and what is commonly found in the literature on this technique.(18,20) Patients defined as clinical responders to DRG stimulation and so implanted with definitive IPG showed a sustained and long term efficacy. Eight patients had previously been implanted with a traditional SCS without any clinically relevant efficacy; they were then explained for unsatisfactory results. Six of them (75%) were later implanted with DRG, with long-term effectiveness. Another advantage of this therapy is the absence of positional effects and lead migration. The adverse events proved to be independent of the anatomical level of insertion; moreover, this series of cases show a lower incidence of lead migration than reported in the literature. In summary, DRGs have been ignored for too long, probably due to the technical difficulty of reaching their deep, almost extra-spinal anatomical position.
1. BACKGROUND
Chronic pain syndromes represent a considerable challenge for pain therapist, despite the most recent developments in the minimally invasive field [1]. Indeed, a high percentage of chronic pain patients are still unable to achieve adequate pain relief with pharmacotherapy, physical therapy, occupational therapy, minimally invasive techniques or surgery [1]. From an economic and social point of view, the impact of chronic pain therefore remains very high [2, 3]. In recent years, a great deal of technological research has been conducted in the field of neuromodulation, in an attempt to provide a solution for patients who are not yet adequately treated, culminating in the identification of promising new stimulation goals, such as the dorsal root ganglion (DRG) [4-7].
DRG, despite its key role in neuromodulation therapy, has been somewhat neglected for years. Recent molecular studies, however, have brought to light the fundamental role of this structure in the origin, development and maintenance of chronic pain [8, 9].
The DRG is involved in the transduction of pain signals to the central nervous system (CNS) and undergoes a series of physiopathological modifications during states of chronic pain. These variations modify the membrane properties of sensory neurons in the first order, and thus their neurophysiological characteristics. Given the alterations in the biophysical properties of these cellular elements, neuromodulatory therapies may preferentially target these elements [10-13].
Spinal cord stimulation (SCS) has been widely used over the last twenty years as an efficient therapeutic option when drug therapies and minimally invasive treatments have proven ineffective in neuropathic pain control [7]. This technique, however, is limited by the need to treat extremely selective and circumscribed areas, such as feet, groin or legs; all these neuronal targets are best covered by DRG selective stimulation [13, 14]. The purpose of this data collection was to evaluate the short- and medium-term safety and efficacy of DRG stimulation for the treatment of chronic pain and its impact on functional aspects.
2. MATERIALS AND METHODS
DRG stimulation with a dedicated device was started in October 2013 at the Department of Pain AO Ospedali Dei Colli - Monaldi Hospital. We retrospectively analyzed the medical records of all patients undergoing DRG Stimulation from the date of the first implantation in October 2013, selecting all patients who have completed a 48-month follow-up. This series of retrospective cases also includes patients where the study failed. For all patients, following the standards of actual clinical practice, the inclusion and exclusion criteria for the implant should be considered which is shown in Table 1.
Always following our real clinical practice for all the patients we used: Visual Analogic Scale (VAS) measuring the intensity of pain Oswestry Scale (ODI) for an estimation of disability at the baseline and 15, and 30 days, and 3, 6, 12, 24, 36 and 48 months after medical intervention/operation. During this period all adverse events that occurred were recorded.
According to the previous criteria, from October 2013 to the end of November 2015, 44 patients with non-reactive chronic neuropathic pain syndrome were selected and subjected to DRG simulation testing. All patients were contacted to provide written consent to participate in this data collection and all information was collected anonymously. A previous failed SCS was recorded for all patients.
Continuous data are presented as an average ± SD. For all the variables collected continuously, the differences before and after the DRG plant were evaluated using ANOVA for the analysis of repeated measurements with the Bonferroni adjustment for post-hoc comparisons. All p values at 2-tail <0.05 were considered statistically significant. The STATUS value ver. 13 statistical package was used for the analysis.
3. SURGICAL TECHNIQUE
One or more quadripolar percutaneous leads were implanted in the posterior roots of the ganglia related to the previously mapped pain area. With local anesthesia plus MAC (Monitored Anaesthesia Care), leads were placed with an epidural approach, with the loss-of-resistance technique. The interlaminar space selected for the epidural approach was one or two spaces lower than the target. The leads are navigated through the epidural space and then placed in the intervertebral foramen near the DRG under the fluoroscopic approach and guidance. The paresthesia is evoked to confirm the correct position of the conductors; the conductors were then connected to an EPG (External Pulse Generator) via test extensions; these were removed at the end of the test period and replaced with the final ones. All patients underwent a trial period to verify the effectiveness of the stimulation. Patients classified as respondents (pain relief >=50%) at the end of the trial phase underwent the final neurostimulator implant (Axium, St. Jude Medical-Plano TX) [15-17].
| Inclusion criteria: |
|---|
| • Therapeutic profile (pharmacological/non-pharmacological) stable over the 30 days preceding admittance, without significant variations in surgical or infiltrative therapy; |
| • Psychological screening has not elicited any contraindications. |
| • The patient has a full understanding of the assessment, implantation, follow-up processes, and risk of complications |
| • Age over 18 years; |
| • Absence of significant comorbidity. |
| Exclusion criteria: |
| • The presence of another clinically significant or disabling persisting pain condition. |
| • A coagulation disorder, immunosuppression, or other conditions associated with an unacceptable surgical risk. |
| • An expected inability to receive or operate the SCS system. |
| • A life expectancy of less than one year. |
| • An expected or planned pregnancy. |
4. RESULTS AND DISCUSSION
Table 2 also specifies the pathologies of the 5 patients indicated as “others”, including the outcome of the study. Compared to a previous neurostimulation procedure, 8 patients had already performed a failed SCS study. (4 for PHN, 3 for CRPS and 1 for FBSS). Of these, only 2 patients, both with PHN, did not respond positively to DRG stimulation. In 44 patients, a total of 72 leads (average of 1.7 ± 0.7 per patient) were placed epidurally at cervical (7%), thoracic (21%), lumbar (58%) and sacral (14%) spinal level. The implant was unilateral in 27 patients (61%). At the end of the trial phase, 39 patients (89%) were shown to be responsive to DRG stimulation and internal pulse generator were implanted, while for the 5 non-responsive patients the leads were explanted.
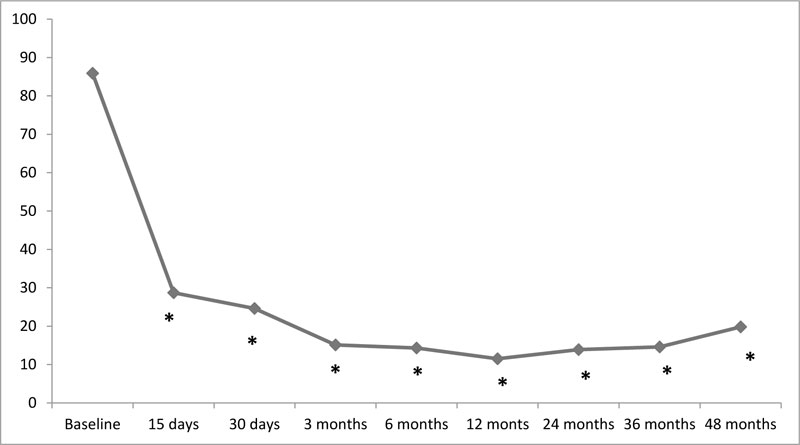
In implanted patients (N=39) the average degree of pain relief was 72% ± 10.1, compared to 11% ± 3.4 in patients who did not undergo the final system implantation (N=5). After the DRG simulation for implanted patients only, the SEA showed a statistically significant reduction during the entire observation period (baseline SEA = 85.9 ± 9.2, VAS15days = 28.7 ± 7. 2, SEA30 days = 24.6 ± 5.2, SEA3 months = 15.1 ± 4.3, SEA6 months = 14.3 ± 4.7, SEA12 months = 11.5 ± 2.7, SEA24 months = 13.9 ± 3.1, SEA36 months = 14.6 ± 5.1, SEA48 months = 19.8 ± 5.9, p = 0.00001). In fact, the SEA scores recorded at each subsequent examination were significantly lower than the basic value (Fig. 1).
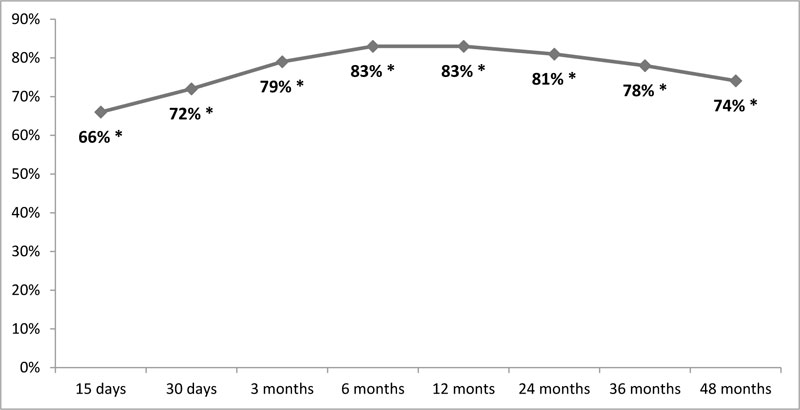
The average pain relief obtained after 48 months of treatment was 74,1% ± 3.4, a percentage absolutely comparable to that obtained at the end of the trial phase. The average percentages during the whole follow-up are shown in Fig. (2).
In addition, the disability index, measured according to the Oswestry Scale, showed a significant improvement during the entire follow-up period (p = 0.00001), with a reduction from 67.5% ± 15.6 at the baseline to 25.9% ± 8.8 after 48 months of treatment. In addition, all scores recorded during follow-up visits were significantly lower than the baseline value (OSW15days = 30.6% ± 7.2, OSW30days = 28.0% ± 7.3, OSW3months = 23.1% ± 8.7, OSW6months = 20.7% ± 9.1, OSW12months = 21.1% ± 8.8, OSW24months = 23.8% ± 7.6, OSW36months = 26.8% ± 8.2, OSW48months = 28.9% ± 7.8) (Fig. 3).
During the follow-up, 9 adverse events occurred, with a complication rate of 20%. Infection was the most frequent side effect, occurring in 3 patients (7%). In all 3 cases, the implant was removed. Two patients underwent implant removal due to rejection. Bilateral lead migration occurred in 1 patient and required an additional surgical procedure. Out of 72 leads implanted, only 2 migrated, with a 3% dislocation rate. All complications occurred within 6 months of implantation, no complications were recorded in the remaining time period. We kept follow-up active for all 34 remaining patients for 48 months.
Considering only the most frequent etiologies (FBSS, CRPS, radicular pain, post-herpetic neuralgia (PHN) and chronic post-surgical pain), a significantly lower percentage of responders (p = 0.041) was recorded among patients with PHN than among the other groups. At the 12th, 24th, 36th and 48th month of follow-up, the reduction in the VAS scale, the pain relief achieved and the improvement in functional aspects were comparable in all the subgroups considered.
| Variable | N | % |
|---|---|---|
| Male | 26 | 64% |
| Age at implant (yrs) | 58±23 | NA |
| Failed Back Surgery Syndrome | 12 (12/0)* | 28% |
| Complex regional pain syndrome | 8 (8/0)* | 18% |
| Radicular pain | 8 (8/0)* | 18% |
| Postherpetic neuralgia | 5 (3/2)* | 11% |
| Chronic postsurgical pain | 4 (4/0)* | 9% |
| Limb phantom/ stump pain syndrome | 2 (2/0)* | 5% |
| Other ** | 5 (2/3)* | 11% |

CONCLUSION
Since the introduction of a dedicated system for DRG stimulation, it was immediately evident how this type of therapy could improve pain relief for patients with chronic neuropathic pain [18-23]. Equally evident is the effectiveness of DRG stimulation in diseases such as CPRS [24-25], FBSS [26-27], phantom limb syndrome [28-30], amputation pain [31-32] and post-surgical pain [33-35]. In particular with regard to CPRS in 2017, Deer et al. in the ACCURATE study highlighted how DRG stimulation significantly reduces the VAS values in these patients, demonstrating to be superior to SCS [36]. With regard to the other indications, there are a number of cases concerning the efficacy of DRG stimulation in postherpetic neuralgia [37-38]. Also for other pathologies, present in our clinical history, such as ischemic neuropathic pain, groin pain, diabetic neuropathy, there is literature to support [39-44]. In this series of cases, these pathologies have been treated mainly by reproducing in real life the results described in the literature [39-44]. Compared to the literature, where the follow-ups are shorter, the respondents in our series maintain pain relief at 48 months.
The results of this data collection demonstrate the feasibility of DRG stimulation, the correspondence between clinical indications to DRG implantation and what is commonly found in literature about this technique [18, 20] Patients defined as clinical respondents to DRG stimulation and then implanted with definitive IPG showed sustained and long-term efficacy. Eight patients had previously been implanted with a traditional SCS without any clinically relevant efficacy; therefore they were explained for unsatisfactory results. Most of these were subsequently implanted with DRG leads, with long-term efficacy. In addition, as with traditional SCS, the procedure is minimally invasive and does not change the operating time or hospital stay. Another advantage of this therapy is the absence of positional effects and lead migration. Adverse events were found to be independent of the level of anatomical insertion. As regards the complications observed in our retrospective analysis, we detect lower rates than the broad case studies analyzed by Huygen et al. These authors observed 11.8% of electrode migrations and dislocations, 10.2% of pain at the pocket site and 5.1% of infections [45]. For these differences in our case studies it is necessary to make some considerations. These patients were operated in the first period of introduction of DRG stimulation systems, for which the first dedicated electrode new to the market was used. It should be remembered that this was equipped with a Tip Ball that significantly reduced its dislocation. The Tip Ball was subsequently removed from production due to difficulties in removing the electrodes. As far as the pain in the pocket site is concerned, it was probably not highlighted in our case studies, since in the operating protocol the IPG was always positioned in the abdominal region. Finally, the infection rate in our series of cases is congruent with the literature. It must be considered that with the passage of time, increased experience reduces the rate of complications. [46, 47].
Our results in maintaining Pain Relief over time are satisfactory. A recent review showed that between 20% and 40% of patients undergoing SCS, pain relief decreases over time due to central nervous system tolerance [48, 49]. Loss of efficacy is the main cause of explantation for SCS systems [50]. In SCS, the activation of the descending pain inhibition system certainly occurs [51]. Taghipour et al. have suggested that stimulation of dorsal column neurons may cause the release of neurotransmitters at the dorsal horn level from the efferent fibers of periaqueductal grey matter (PAG), and rostral ventromedial marrow (RVM), with antinociceptive effects [52]. Another study proposes the following mechanisms to explain the effects of SCS on chronic pain [53]. In chronic neuropathic pain, the mechanism of disinhibition of the dorsal horn circuits that would allow the Aß fibers to access the lamina I neurons would be particularly increased. SCS would amplify the anti-dromic impulses of the Aß fibers by energizing the lamina I fibers in the dorsal horn.
DRG stimulation unlike SCS acts directly on the primary sensory neurons of the DRG, which represent an important target in the pathophysiological mechanism of many types of neuropathic pain [54]; the DRG contains the primary sensory neuron (PSN) cell bodies and their T-junctions. It is precisely in the T-junction that the failure of the central projection of sensory information can be jammed, just as in this area modulation occurs for the sensory control of peripheral stimuli, especially painful ones [55-57]. Morgalla et al. have hypothesized that DRG stimulation can normalize, maintaining this effect indefinitely over time, the transmission of painful rye from the periphery to the supraspinal levels [58].
Despite the current dominance of conventional SCS, stimulation peripheral nerves stimulation has again attracted the interest of pain therapists over the past two and a half decades. Thus, nerve roots and the brain have again become the main focus of interventions for the treatment of pain (basal ganglion and thalamus) and psychiatric disorders (internal capsule, cortical and subcortical regions). In summary, DRGs have been ignored for too long, probably due to the technical difficulty of reaching their anatomical position. In conclusion, we believe that this technique should always be taken into consideration, especially in cases where SCS does not give optimal results, despite the technical difficulties of implantation.
AUTHORSHIP STATEMENTS
AP defined this data collection and interpreted the data. AP also analyzed the data and drafted the manuscript. The other authors collected the data and reviewed the manuscript. All authors approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
In our hospital, the approval of the Ethics Committee is not required for a retrospective observational study for a case series; for this reason no request was made.
HUMAN AND ANIMAL RIGHTS
Not Applicable.
CONSENT FOR PUBLICATION
All patients have signed consent for data publication.
STANDARDS OF REPORTING
Clinical practices were carried out according to standard of CARE; no changes to common clinical practice occurred for the treatment of these patients.
FUNDING
No funding was obtained for this study.
CONFLICT OF INTEREST
AP is the consultant (proctor) to St Jude Medical/ABBOTT. Other Authors have nothing to disclose.
ACKNOWLEDGEMENTS
Declared none.


