High-Tone External Muscle Stimulation for the Treatment of Chronic Sciatica – A Randomized Controlled Crossover Trial
Abstract
Background:
Chronic sciatica is a common pathology with a lifetime prevalence of 84%. Current therapy options are inadequate or not long-lasting.
Objectives:
Evaluation of short-term application of High-Tone Electrical Muscle Stimulation (HTEMS) compared to Transcutaneous Electrical Nerve Stimulation (TENS) with chronic sciatica.
Methods:
Patients (n=100, (mean±SD) age=57±14 years, sex=42% male) with chronic sciatica were randomly assigned into two groups treated with either HTEMS or TENS. Each treatment was administered for a period of 45 min per day, 5 times within 7 days, with a 7-day wash-out period before crossover. A 5-day average of sciatic pain was assessed using the visual analog scale (VAS) before and after intervention. Drug administration was stable during the study.
Results:
Before crossover, pain intensity was significantly reduced by the HTEMS treatment (56±21 (60 [50-70]) to 45±21 (50 [30-60]) mm VAS; p<0.001), while no improvement occurred with TENS (59±19 (60 [50-70]) to 56±19 (60 [45-79]) mm VAS). After crossover, significant pain reduction was observed in both groups (both p <0.01) and did not differ between both groups after the whole intervention.
Conclusion:
HTEMS showed a higher potential for short-term reduction of pain than TENS and might offer new a therapeutic strategy for the treatment of chronic sciatica.
1. INTRODUCTION
Low back pain is a very common ailment that tends to affect most people with varying degrees of symptom severity. Low back-related leg pain or sciatica is one of the most common variations. Sciatic pain radiates from the low back or buttock to below the knee and can be accompanied by clinical findings such as herniated disc or nerve root irritation [1, 2]. Chronic sciatic pain is a common reason for patient visits and hospitalization to a health care provider or physician [3]. The lifetime prevalence was shown to be as high as 84%, and the prevalence of pain chronification is about 23%, with 11-12% of the population being disabled [4]. Chronic sciatica and low back pain are the leading causes of disability for patients younger than 45 years [5] and represent collectively the third most common cause for surgical procedures [3]. The costs associated with sciatic pain include the direct costs of medical care and indirect costs for time lost from work, disability payments, and diminished productivity [6].
When sciatica is not associated with neurologic deficits, like bladder or bowel dysfunction, conservative therapy is indicated. This includes systemic or local drug administration, physical therapy and chiropractic treatment beside psychotherapy, psychoeducation and psycho-social support [7]. When conservative treatment fails, elective surgery should be considered for those with functional disabilities or refractory pain who are unresponsive to conservative therapy [7, 8]. Nevertheless, despite those therapeutic options a large number of patients keep on suffering from chronic sciatic pain, remain unable to work, and have persistent symptoms [9, 10]. Several forms of electrical stimulation are used for non-pharmacological pain treatment. All methods are based on the hypothesis that electric stimulation inhibit pain transmission and also might enhance microcirculation [11]. TENS is the most frequently used method with the potential to decrease pain [12, 13], although overall results are controversial [14], especially in the context of chronic low back pain [15].
Based on observations that simultaneous modulation of frequency and amplitude improves pain, High-Tone External Muscle Stimulation (HTEMS) had been developed [11]. HTEMS delivers much more energy through skin surface electrodes into tissues compared to TENS. In contrast to classical electrotherapy, HTEMS uses alternating electric fields to stimulate different tissues. Furthermore, the current intensity and the frequency can be modulated at the same time. In a short comparative study, we demonstrated that HTEMS was almost three times more effective than TENS in reducing pain and discomfort in patients with painful diabetic neuropathy [16]. Based on this finding, we conducted another intervention study over 12 weeks in diabetic patients with Peripheral Neuropathy (PNP) suffering from moderate symptoms of PNP. In this study, we demonstrated that long-term application of HTEMS leads to clinically relevant improvements in symptoms of diabetic PNP (e.g. reduction in pain) [17]. However, no data is available on the effectiveness of HTEMS for the treatment of other pain-associated indications. Therefore, the aims of the present study were to analyze in a proof of concept study the immediate pain-relieving effect of HTEMS on chronic sciatic pain and, furthermore, to compare this effect with TENS in a randomized controlled crossover trial with patients suffering from chronic sciatica.
2. MATERIALS AND METHODS
2.1. Study Population
Patients suffering from severe attacks of chronic sciatica and requiring hospital treatment at the Spine Unit and Center of Pain Management of the St. Vinzenz Krankenhaus, Düsseldorf, Germany, were invited for participation in the present study. Informed written consent was obtained from all patients prior to inclusion into the trial (ClinicalTrial.gov registration no: NCT02151565) which was in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Approval of the research protocol was obtained from the ethics committee of the Ärztekammer Nordrhein, Düsseldorf, Germany. In the present study, eligible patients (n=100) are characterized by the following inclusion criteria: (i) chronic sciatica, (ii) continuously existent for at least 3 months, (iii) which was accompanied by degenerative lumbar spine disorders with a documented Magnetic Resonance Imaging (MRI) or Computed Tomography (CT) finding that associates with the symptoms (without severe neurological impairments) and (iv) with stable oral analgesic regimen. Exclusion criteria comprised: (i) a history of drug or alcohol abuse, (ii) symptoms or signs that favor surgical interventions such as paralysis or bowel//bladder dysfunction, (iii) oncologic pathologies requiring intense analgesic regime adjustments, (iv) cardiac pacemaker or defibrillator, (v) pregnancy or (vi) acute thrombosis, bacterial infection and fractures. Eligible patients were randomized with a 1.5:1 (HTEMS treatment in the first phase (H1T2) n=59; TENS treatment in the first phase (T1H2) n=41) ratio into two groups using an electronically generated randomization list (generated by the trial statistician) (Fig. 1). We have chosen this approach as the present study is a 2-armed confirmatory/hypothesis-testing trial aiming to investigate the treatment effect of HTEMS in patients with chronic sciatica as well as to search for adverse events [18]. In detail, each participant was assigned a serial study identifier (ID). For each ID, there was a closed envelope with group assignment. Patients were enrolled by the trial physician during a period of 12 months. The first participant was enrolled in the study in November 2012 and the last patient finished the intervention in November 2013. Treatment and data collection were not blinded for the patients and the trial physician but blinded for the trial statistician.

2.2. Study Design
Study participants were randomly assigned to two groups. The first group (H1T2) is characterized by an initial intervention with HTEMS that is followed after crossover and wash-out phase by the second intervention period with TENS. The second group (T1H2) started initially with the TENS treatment that was followed after crossover and wash-out phase by the HTEMS treatment. Each single treatment session lasted for 45 min per day, 5 times within 7 days, with a wash-out period of 7 days before crossover. Pre-intervention treatment with analgesics, especially morphine and pregabalin, was allowed and had to be stable during the study period. Drug adjustments during the study led to study exclusion.
2.3. Treatment
Cutaneous electrode pads were applied to the corresponding myotomes of the affected low back area with peak pain in both treatment arms. The HTEMS device HITOP 191 (gbo Medizintechnik AG, Rimbach, Germany) generated pulse widths of ≤350 mA, ≤70 V with an initial frequency of 4,096 Hz that were increased over 3 sec to 32,768 Hz, held at maximum for 3 sec and then down modulated to the initial frequency. For each participant, the intensity was adjusted to a level that did not produce any pain or discomfort as described elsewhere [16, 19]. HTEMS and TENS treatment was conducted at the same time on each day taking circadian rhythm of pain into account [20].
TENS treatment was performed with the H-Wave device Dumo 2.4 (CEFAR Medical, Lund, Sweden), a portable and rechargeable unit that generates a biphasic exponentially decaying wave form with pulse widths of 4 msec, ≤35 mA, ≤35 V and 100 Hz. Intensity was adjusted according to the patient’s pain perception and ranged from 20 to 30 mA as described elsewhere [16, 19].
2.4. Pain Evaluation
Sciatic pain, i.e. an average pain over 5 days, was the primary study outcome and had been assessed using a 100 mm Visual Analog Scale (VAS) ranging from 0 mm, representing no pain, to 100 mm defining the worst pain imaginable. The measurement was conducted before and after each treatment session. The VAS measurement has already demonstrated its sensitivity to changes in pain in other studies. Furthermore, the VAS can detect hourly and weekly pain changings following pain therapy in a broad range of populations [21].
2.5. Statistical Analysis
Data are presented as mean ± Standard Deviation (SD) and in brackets as median with inter-quartile range, or Standard Error of the Mean (SEM) as well as percentages. For non-parametric data, Mann-Whitney U test, Fishers exact test as well as Wilcoxon signed rank test and for parametric data, Student’s t-test or paired t-test were applied to determine differences between groups and changes following the intervention. Multivariable linear regression analyses were performed to investigate the influence of the current drug treatment on changes after HTEMS or TENS adjusted for the initial pain value on the VAS. Sample size had been calculated assuming that HTEMS improves pain by 15 ± 14 mm VAS, while for the control group a reduction of only 5 mm VAS was estimated. Our assumption based on previous work [16, 17, 19]. To be able to measure such a difference with a power of 90% and a level of significance of 5%, at least 42 datasets per group would be needed. Since a dropout rate of about 20% was estimated, the plan was to recruit a total of 100 persons. Intention-to-treat analyses were performed, and missing values were substituted by the ‘last-observation-carried-forward’ principle. Group allocation had been blinded to the outcome assessor. Treatment and data collection were not blinded for the patients and the trial physician. Level of significance was set at α=0.05. Statistical analyses were performed using GraphPad Prism 6.04 (GraphPad Software, San Diego, CA, USA) and SAS statistical package version 9.3 (SAS Institute, Cary, NC, USA).
3. RESULTS
The demographic and clinical characteristics of the groups studied are shown in Table 1. Fifty-nine patients were in the H1T2 group (age: 57±14 years) and 41 patients in the T1H2 group (age: 57±13 years) (Fig. 1). The two groups did not differ in any of their baseline characteristics (Table 1). Furthermore, both groups were characterized by moderate chronic sciatic pain before starting the intervention (mean: 56-59 mm VAS). All patients finished the first phase intervention, but four patients refused to start the second intervention phase after crossover. One patient in the H1T2 group refused to use TENS, two patients were free of pain after HTEMS intervention and one patient in the T1H2 group reported pain increase after TENS intervention. Another 2 patients dropped out during the second phase of intervention because they refused to participate at the TENS treatment. Fifty-four patients of the H1T2 group and 40 patients in the T1H2 group finished both intervention phases. The individual analgesic drugs/drug classes for both groups are listed in Table 1 as well as the distribution of degenerative lumbar spine disorders and leg-pain associated pathologies (such as diabetes mellitus and polyneuropathy).
| – | H1T2 (n=59) | T1H2 (n=41) |
|---|---|---|
| Sex (male/female) [n] | 28 (47%) / 31 (53%) | 14 (34%) / 27 (66%) |
| Age [years] | 57±14 | 57±13 |
| VAS [mm] | 56±21 | 59±19 |
| NPP [n] (%) | 26 (44%) | 17 (41%) |
| Spinal canal stenosis [n] (%) | 10 (17%) | 11 (27%) |
| Degenerative bone disease 1 [n] (%) | 34 (58%) | 21 (51%) |
| Sakroiliopathy [n] (%) | 3 (5%) | - |
| Diabetes mellitus [n] (%) | 2 (3%) 2 | 4 (10%) 2 |
| Polyneuropathy [n] (%) | 2 (3%) 2 | -2 |
| Treated with morphine [n] (%) | 41 (69%) | 24 (59%) |
| Treated with pregabalin [n] (%) | 27 (46%) | 21 (51%) |
3.1. Significant Pain Reduction During HTEMS Intervention
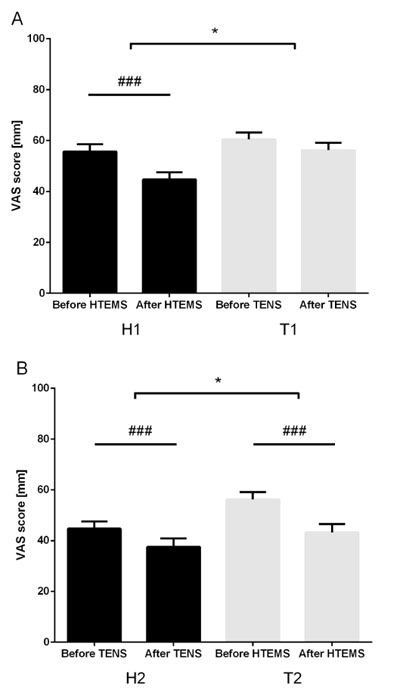
During the first phase of intervention mean pain intensity was significantly reduced from 56 ± 21 (60 [50-70]) to 45 ± 21 (50 [30-60]) mm on the VAS in the H1T2 group (p<0.001), while no statistically significant improvement occurred in the T1H2 group (change from 59 ± 19 (60 [50-70]) to 56 ± 19 (60 [45-79]) mm on the VAS; (Fig. 2A). Fifty-six percent of the participants in the H1T2 group reported a pain improvement of at least 10 mm on the VAS, while only 41% of the T1H2 group reported such a pain reduction (p=0.047). The Odds ratio [95% confidence interval] was 1.83 [1.05-3.21] for HTEMS compared to TENS to achieve at least 10 mm on the VAS. The treatment effect of a short-term application of HTEMS was a reduction of 10 ± 17 (10 [0-20]) mm (p<0.001) and 4 ± 15 (0 [0-10]) (n.s.) mm with TENS in pain intensity on the VAS during the first phase of the intervention that significantly differed between both groups (p=0.046). After crossover and wash-out period, a reduction of 13 ± 15 (10 [0-20]) mm VAS (p<0.001) with the HTEMS treatment and 7 ± 14 (10 [0-20]) mm VAS (p=0.0015) with the TENS treatment was achieved. HTEMS treatment of the T1H2 group induced during the second phase a significantly higher pain reduction than the first phase treatment with TENS (p=0.0075) as shown in Fig. (2B). Looking at the total pain reduction after both intervention series there was no statistically significant difference between H1T2 and T1H2 (Fig. 3A). However, the difference of pain reduction after the individual intervention series demonstrated a higher pain improving potential of HTEMS compared to TENS (p=0.011) as shown in Fig. (3A and S1). Number or doses of drug treatment during the intervention phases had no statistical influence on the treatment effect of TENS or HTEMS (data not shown).
4. DISCUSSION AND CONCLUSION
The results of this proof of concept study demonstrate that a short-term intervention over 5 days with HTEMS has the potential to immediately reduce sciatic pain with a significantly stronger analgesic effect than TENS in middle-aged patients with chronic sciatica. Particularly, the first period of intervention showed that HTEMS reduces pain to a greater extent than TENS (mean reduction: absolute: 10 mm; relative: 18%), although the reduction of pain level resulted not in a clinically relevant extent [21-23]. But from a clinical point of view, HTEMS showed a promising result, especially when comparing with TENS or considering the short period of intervention time. Most of the studies, which had led to a clinically relevant reduction in this context (i.e. ≥30% or ≥20mm VAS change), comprised a study duration ranging from 5 to 12 weeks [24]. Regarding TENS therapy, previous work underpins our results stating that TENS is not recommended for the treatment of chronic low back pain or associated pathologies [25]. Furthermore, patients of the initial HTEMS group (H1T2) almost switched within the pain scale classification from moderate to mild pain (56 to 45 mm) [21]. In patients with moderate chronic low back pain ranging from 45-74 mm on the VAS, improvements of at least 20-25 mm (absolute) or 30-33% (relative) were stated as the threshold for a minimal clinically important difference [23, 26]. This threshold was reached after 6 months of multifactorial therapy in patients with chronic low back pain [26, 27].
There is controversy concerning the effectiveness of conservative and pharmacological treatments. Although a large variety of non-surgical strategies is available for the management of chronic sciatic pain, most treatments are associated with delayed recovery or inconsistent results. However, acupuncture, exercise therapy, multifactorial programs, epidural steroid injections and spinal manipulation can be effective in sciatic and low back pain, but studies have demonstrated mixed results [8-10].

Like physiotherapy and isometric exercise, HTEMS and TENS are non-invasive and without any negative side effects when performed properly. Even during pregnancy studies indicate that electrotherapy against pain has no adverse effects on birth or child well-being [28]. This is an enormous advantage compared to pharmaceutical therapies or invasive methods such as injections and surgery. The effectiveness of HTEMS might be based on neurophysiologic and neurochemical mechanisms that are stimulated by the electrotherapy [11, 29]. Although the exact mechanisms are unknown, so far it was postulated that HTEMS enhances the release of endogenous analgesics [30]. Additionally, it increases vasodilatation (enhanced bioavailability of nitric oxide) leading to improved microcirculation and endoneural blood flow (locally and systemically) [11, 29, 30]. Furthermore, it is assumed that the inhibition of sympathetic afferent activity decreases the pain transmission to brain [31]. Another important assumption is that the application of neuromuscular electrical stimulation improves muscle strength contributing to an improved state of motor control of the spine as it was also shown after motor control exercise [29, 32]. As consequence, one could speculate that the treatment difference between both groups (HTEMS vs TENS) results from the mimicking effect of HTEMS that can be seen after regular exercise [32].
Since we intended to analyse the effects of HTEMS and TENS on immediate pain reduction with 5 applications within 7 days, with maximal one treatment per day, in patients with chronic sciatic pain we cannot speculate on long-term effects. For such an analysis, patients would have to be continuously treated after their hospital stay. Moreover, one might argue that we could not exactly distinguish between effects of electrotherapy and analgesic medication. However, treatment with analgesics, especially morphine and pregabalin has been started before the beginning of electrotherapy and the doses had not been changed during the study phase. Furthermore, the number and the doses of drug treatment during the intervention phases had no statistical impact on the treatment effect of TENS and HTEMS. Therefore, we exclude a pharmaceutical influence on the reported pain reduction. Future studies should, as it is one major limitation of our trial, include patients not already treated with opioids to prevent a potential mixture of analgesic and electrotherapy effect. Furthermore, information about the history and duration of pain or medication as well as psychological aspects had not been available, although those factors could have led to an impact on the treatment results. Moreover, we cannot explain our results on the basis of electroneurophysiological measurements, such as electromyography (EMG) or measurement of Nerve Conduction Velocity (NCV), and, therefore, future HTEMS studies should chose a more mechanistic approach. Another limitation is that, although the treatment and data collection was blinded to the trial statistician, there is a possibility for a selection bias regarding the data due to unblinded patients and treating clinicians. Moreover, there is the possibility for a carry-over effect since the pain-relieving effect of the initial treatment did not vanished following the washout period. However, the lasting pain reduction could be a “period effect” that occurred because of factors unrelated to the treatment effect such as an interaction with the ongoing analgesic treatment or seasonal effects [33]. Furthermore, a pre-test we had performed for the calculation of a possible carry-over effect showed that the washout phase was long enough (data not shown) [34]. Apart from that, the strengths of our study comprise that: (i) we included a relatively large number of participants, (ii) we conducted a RCT with (iii) a crossover design allowing for an internal and external control group comparison regarding different effects of HTEMS and TENS. Although the 10 mm VAS pain reduction is not clinically relevant in the present study, at least we can rely on the beneficial effect of the HTEMS treatment compared to the TENS approach based on our sample size calculation. However, future studies should replicate our trial with a longer duration and more participants to confirm our promising results and to investigate for long-term and clinically relevant effects. In sum, this is the first randomized controlled crossover trial that analysed the effect of HTEMS on the immediate pain relief in patients with chronic sciatic pain. Our results demonstrate that a short-term intervention over 5 days with HTEMS reduces pain and the effects were significantly stronger compared to TENS therapy. These findings must be replicated in long-term interventions but indicate that HTEMS could be a new therapeutic option for the treatment of chronic sciatica.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Approval of the research protocol was obtained from the ethics committee of the Ärztekammer Nordrhein, Düsseldorf, Germany.
HUMAN AND ANIMAL RIGHTS
The reported experiments are in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931
CONSENT FOR PUBLICATION
Informed written consent was obtained from all patients prior to inclusion into the trial (ClinicalTrial.gov registration no: NCT02151565).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
M.R. and K.K. wrote the manuscript, researched and collected data. M.R. and K.K. performed the statistical analysis. S.M. had the idea, initiated the study and revised the manuscript. E.D., J.H., S.J. and S.SZ. researched data and contributed to the manuscript. S.M. is the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.