Ultrasound-guided Perineural Dextrose Injection for Treatment of Superior Cluneal Nerve Entrapment: Serial Case Report
Abstract
Background:
Superior cluneal nerve entrapment is a neuropathic condition caused by the inclusion of the superior cluneal nerve that contributes to one of the causes of lower back pain leading to high morbidity. Several therapeutic modalities
are available for superior cluneal nerve entrapment, including medications, physiotherapy, perineural injection, and surgery. Perineural injection with 5% dextrose has become therapeutic alternative in many cases of neuropathy, but its long-term effectiveness is unknown.
Case Presentation:
This study described four patients with superior cluneal nerve entrapment with severe pain intensity treated with ultrasonography guided perineural 5% dextrose injection, resulting in significant clinical improvement during the 6-month evaluation.
Conclusion:
Perineural injection can be considered as long-term therapy in patients with superior cluneal nerve entrapment who have failed other conventional therapies.
1. INTRODUCTION
Low Back Pain (LBP) is a major health problem leading to high morbidity worldwide. Approximately 50-80% adults reported having experienced LBP during their lifetime [1]. One of the causes is Superior Cluneal Nerve entrapment (SCN-E), a neuropathic condition due to SCN entrapment at the iliac crest, which contributes to 14% of all LBP cases worldwide [2]. The average age of onset of SCN-E ranges from 55 to 68 years, and women comprise 55-63% of all cases [3]. LBP due to SCN-E is exacerbated by various types of lumbar movement, and its features are fully elucidated, often leading to a misdiagnosis of lumbar spine disorder [2, 4-6].
Perineural injection with 5% Dextrose (D5W) was developed as a therapeutic option in many cases of neuropathy because of its good anesthetic and analgesic effects, with minimal risk of anesthetic toxicity [7]. Chang et al. have reported 80% pain relief for 2 weeks after a combination of D5W and lidocaine in one case of SCN-E [8]. The Maniquis-Smigel study has compared the administration of D5W to Normal Saline (NS) in chronic cases of LBP. This study resulted in a significant reduction in the Numeric Rating Scale (NRS) of 84% (p <0.001) in the first 4 hours post-injection [9]; however, the long-term effectiveness of D5W therapy has not been widely discussed, especially in SCN-E cases. Therefore, four cases of SCN-E with severe pain intensity that were treated with an Ultrasonography (USG)-controlled perineural D5W injection and experienced significant clinical improvement at 6-month of evaluation were presented in this study.
2. CASE
Four patients, including two women and two men aged 40, 42, 47, and 75 years, respectively, complained of LBP. Three patients showed acute LBP, whereas the other experienced chronic LBP. Two patients experienced a burning sensation in the unilateral buttocks radiating to the ipsilateral upper thigh, whereas the other two experienced unilateral LBP on the unilateral hip and pelvis without any radiating pain. In all patients, worse pain was reported when certain changes in position were made, such as bending over, walking, and sitting, with a pain scale of 8/10 to 9/10. In addition, none of the patients had symptoms of hemiparesis, numbness, or tingling sensations. There was no history of trauma, tumor, or surgery in the pelvic area. One patient had a history of Herniated Nucleus Pulposus (HNP) at the right lumbar level of 4-5 for ten years. Meanwhile, one other patient had been lifting heavy loads as a work risk. Four patients received oral medication therapy for seven days, but there was no improvement. On clinical examination, a trigger point reported at the top of the unilateral medial iliac crest (Table 1).
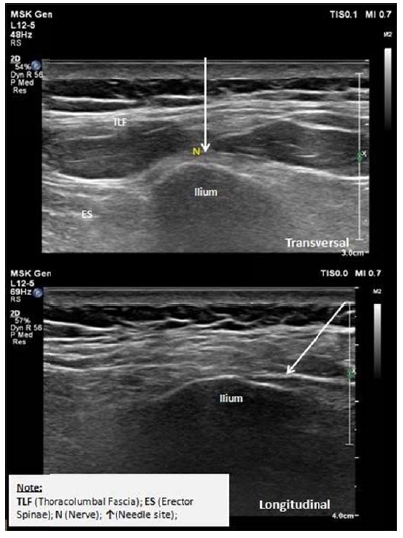
In all four patients, the diagnostic blocks were performed with 2% lidocaine. Fifteen minutes after injection, all patients showed significant clinical improvement with a reduction in the pain scale from 2/10 to 3/10. With the consent of each patient, USG-guided perineural injections were performed using 10 ml D5W (referred to in previous studies). The hydrodissection injection method using ultrasound as a guide is performed using the in-plane approach; the thoracolumbal fascia located above the nerves was subjected to hydrodissection (Fig. 1). The neural hydrodissection procedure was performed twice at 2-week intervals. Between both procedures and follow-up, all patients were administered 500 mg acetaminophen thrice daily orally (if necessary). But none reported undergoing any additional care nor needing analgesics provided daily basis. The results enabled a significant resolution in pain, and after the injection procedure was completed, periodic evaluations were performed at 1, 3 and 6 months post-injection. No adverse effects were observed in any of the four patients (Tables 2 and 3).
| Case | Age (year), Gender, Occupation | History | |||||
|---|---|---|---|---|---|---|---|
| Location of Pain | Onset (weeks) | Pain Characteristic | Medication | Physical Examination** | |||
| Oral | Diagnostic Block* | ||||||
| 1 | Mrs. H, 47. Women, Housewive | Left side buttocks | 8 | Radiating to the left thigh, worsen with position changes, NRS 9/10 | 7 days | (+) NRS 3/10 |
TP(+) left medial iliac crest |
| 2 | Mrs. DM, 42, Women, Employee | Left side buttocks | 1 | Radiating to the left thigh, worsen with position changes, NRS 9/10 | 7 days | (+) NRS 3/10 |
TP(+) left medial iliac crest |
| 3 | Mr. X, 40, Men, Mechanic | Right side hip |
1 | Worsen with position changes, NRS 8/10 | 7 days | (+) NRS 2/10 |
TP(+) right medial iliac crest |
| 4 | Mr. S, 75, Men, Retired |
Right side waist |
1 | Radiating to the right buttock, NRS 8/10 | 7 days | (+) NRS 2/10 |
TP(+) right medial iliac crest |
| Case | NRS Baseline Preinjection 1 | Post Injection | |||
|---|---|---|---|---|---|
| 15 minutes | 2 weeks | ||||
| NRS | Complication | NRS | Complication | ||
| 1 | 9/10 | 5/10 | - | 4/10 | - |
| 2 | 9/10 | 5/10 | - | 3/10 | - |
| 3 | 8/10 | 3/10 | - | 2/10 | - |
| 4 | 8/10 | 4/10 | - | 3/10 | - |
| Case | NRS Baseline Preinjection 2 | Post Injection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 minutes | 2 weeks | 1 month | 3 month | 6 month | |||||||
| NRS | Complication | NRS | Complication | NRS | Complication | NRS | Complication | NRS | Complication | ||
| 1 | 4/10 | 3/10 | - | 2/10 | - | 0/10 | - | 0/10 | - | 0/10 | - |
| 2 | 3/10 | 2/10 | - | 0/10 | - | 0/10 | - | 0/10 | - | 0/10 | - |
| 3 | 2/10 | 1/10 | - | 1/10 | - | 0/10 | - | 0/10 | - | 0/10 | - |
| 4 | 3/10 | 1/10 | - | 1/10 | - | 0/10 | - | 0/10 | - | 0/10 | - |
3. DISCUSSION
SCN is a pure sensory nerve that dominates the sensory perception in the lumbar and buttock area [2]. Furthermore, it is made up of three branches: medial, intermediate, and lateral branches that pass through the osteofibrous tunnel formed by the thoracolumbar fascia and iliac crest [2, 10]. An increase in paravertebral muscle tone can increase the tension of the thoracolumbar fascia causing spontaneous entrapment of the SCN [11]. An increase in paravertebral muscle can occur due to other diseases, such as vertebral fractures, lumbar disc herniation, lumbar canal stenosis, Parkinson's disease, post-lumbar surgery, or heavy physical activity as in athletes and soldiers [2, 4, 12-14]. This study describes four cases of SCN-E, consisting of one case of subacute LBP and the other three cases of acute LBP. Two out of three patients with acute LBP had risk factors for SCN-E in the form of a history of lumbar HNP (Mr. S) and strenuous activity as a workshop assistant (Mr. X).
The underlying inflammation that occurs in SCN-E is caused by nerve irritation. The noxious stimulus stimulates the potential of the transient receptor potential vanilloid subtype 1 (TRPV-1), which causes depolarization and production of substance P, glutamate and calcitonin gene-related peptide (CGRP) that destroy nervous tissues [5]. This can cause LBP, which radiates to the posterior thigh, calf, and leg mimicking sciatic pain [3]. In a study of 52 SCN-E cases, 23 patients (44%) had LBP alone, whereas 29 (56%) experienced radiating pain to the lower leg [15]. The pain was exacerbated by several lumbar postures and movements such as extension, bending, rotation, prolonged standing or sitting, walking, and rolling [16, 17]. This study corresponds to the characteristic of neuropathic pain in all four cases.
The diagnosis of SCN-E is based on the clinical manifestations and the diagnostic block of SCN [6]. Local anesthetic injection acts as one of the diagnostic criteria for SCN-E (Table 4) to rule out other possible causes of LBP, such as lumbar HNP, gluteal muscle spasm, facet, and sacroiliac joint disorders [6, 17]. In this study, diagnostic blocks using a USG-guided 2% lidocaine injection were performed to confirm the diagnosis of SCN-E. In all four patients, there was a significant reduction in VAS score from 2/10 to 3/10 within 15 min post-injection, which supported the diagnosis of SCN-E.
| S.No | - | |
| 1 | Low back pain involving the iliac crest and buttocks | |
| 2 | Symptoms aggravated by lumbar movement or posture | |
| 3 | Trigger point over the posterior iliac crest corresponding to the nerve compression zone | |
| 4 | Patients report numbness and radiating pain in the SCN area (Tinel’s sign) when the trigger point is compressed | |
| 5 | Symptom relief by SCN block at the trigger point | |
Several therapeutic modalities are available for SCN-E, including oral medications, physical therapy or physiotherapy, perineural injection therapy, and surgery. However, block therapy, neurolysis, and decompression surgery were the most common therapy used [2]. The choice of therapy in each case varies, based on the intensity of the pain, social impact, disrupted work, and patient consent [18, 19]. Within several case studies related to neuropathy entrapment, the perineural D5W injection has emerged as an alternative therapy for SCN-E. Nerve hydrodissection is a perineural injection technique that uses fluids to separate the nerve from the surrounding soft tissue/fascia. D5W is the most commonly used fluid for perineural hydrodissection. This is because it is harmless to the nerve tissue, surrounding blood vessels, and soft tissue, and has good anesthetic and analgesic effects [20]. Therefore, in all four patients, the perineural injection was administered using D5W.
Although the analgesic mechanism provided by D5W is uncertain, several studies have described D5W effects at several levels, such as its direct effect on pain receptor, sugar levels in nerve cells and surrounding areas, substance P, and CGRP, and mechanical effects due to the release of nerve adhesions [21-23]. In all four patients, the perineural injection was administered twice at 2-week interval, and all injections were guided by USG. Moreover, 10 ml of D5W was used for each injection, and according to Maniquis-Smigel, NRS scores improved by 4 to 5 points 15 min after the first injection [9]. Immediate resolution may be related to the direct effect of D5W in the inhibition of TRPV-1 activation on peripheral nerves, therefore blocking inflammatory cascade [22]. In addition to reducing inflammatory neuropathy, hydrodissection also plays an important role in releasing soft tissue adhesions, therefore repairing nerve impulses and preventing direct ischemic-induced nerve damage [24].
After 2 weeks, all patients reported a decrease in NRS scores ranging from 2 to 4/10 NRS. The second injection procedure was performed using a similar composition and technique to increase the initiation of proliferation. The use of dextrose was not recommended at high concentrations because it can lead to an inflammatory reaction [25]. Periodic evaluations were performed in all patients, resulting in satisfactory pain resolution. Two weeks after the second injection, all patients experienced a significant decrease in the NRS scores to 0-2/10. This is consistent with the study conducted by Chang et al¸ which reported 80% symptom improvement after the USG-guided injection of 1% lidocaine and D5W combination [6, 8]. However, further studies are needed to determine the difference in the use of D5W and local anesthetic combination compared to D5W alone in the case of SCN-E. In addition, the chronic-onset case had the highest NRS score compared to the other case. The possibility of chronic inflammation that occurs may be the cause but should be further investigated.
On the rating of 1,3, and 6 months after the second injection, four patients had complete resolution of pain-related NRS scores of 0/10. This resolution may be due to the D5W effect on peripheral glycopenia [23]. Dextrose injection improves the state of glycopenia, affecting sodium-potassium pumps and C fibers, thereby reducing neuropathic pain rapidly [26]. In addition, D5W also binds to the presynaptic calcium channels, inhibiting the release of substance P and CGRP, leading to a reduction in inflammatory neuropathy. This also leads to an improvement in the flow of nerve growth factors, which triggers nerve tissue repair, and reduces pain [27]. Local side effects, such as redness, infection, swelling, hematoma, weakness, or paraesthesia during the evaluation were not observed in four patients.
Other fluids that can be used for perineural injection include local anesthetics, NS, corticosteroids, and platelet-rich plasma (PRP). In addition to its function as a support in diagnosing SCN-E, perineural injection with local anesthetics can also be used as block therapy. However, no study has documented its long-term effectiveness [4]. Moreover, the repetition of local anesthetics injections over a short period of time is not recommended because it can lead to complications ranging from local adverse effects, such as numbness, paresthesia, peripheral neurotoxicity (prolonged motor and sensory deficits), and muscle tissue damage to systemic toxicity [4, 17]. NS can also be used to enlarge the perineural space, but it has no therapeutic effect on the damaged nerves. Corticosteroids are useful in reducing neurogenic inflammation, but using the substance can cause skin depigmentation, subcutaneous atrophy, and local infection at the injection site [14]. PRP contains a high concentration of autologous growth factors, which are generally used to accelerate tissue repair in musculoskeletal disorders [28]. In contrast to D5W, which contains a definite dextrose concentration, the platelet concentration of PRP varies in each patient and preparation system. Additionally, the increased inflammatory cytokines expression in peripheral nerves causes the injection of leukocyte-rich PRP to increase neurogenic inflammation exacerbating symptoms [29].
So we concluded that there are three proposed mechanisms of how D5W can improve neuropathic pain. First is through its action at key pain modulator level, specifically TRPV-1 ion channel. Upregulation of this channel has been associated with neuropathic pain and certain monosugars (including D5W and mannitol) have been observed to modulate the effect of TRPV-1 expression in an allosteric manner. Secondly, D5W injection has been proposed to correct local glycopenia in peripheral nerve, which has been observed to cause damage. This may be due to the nature of peripheral nerves that are particularly sensitive to glycopenia. Third, dextrose injection may cause hyperpolarization of nerves from the elevation of extracellular dextrose level, which is still investigated thoroughly [30].
Decompressive surgery and neurolysis are also considered options in the treatment of SCN-E, but they are invasive and can lead to complications, such as postoperative scarring leading to nerve disorders [31]. In previous studies, the surgical approach was effective in reducing pain, although some subjects reported symptom reoccurrence and underwent repeated surgery [4].
Compared to other imaging modalities (fluoroscopy, CT, MRI), USG has more advantages, such as an absence of radiation, real-time visualization from placement to needle injection, cost-effectiveness, and availability in healthcare facilities [32]. The use of USG has not only increased the success rate but also reduced the risk of toxicity and complication of perineural injection [8, 33, 34].
The present four case reports do not demonstrate the overall effectiveness of a therapy, but this case series can serve as a reference for future studies aimed at assessing the long-term effectiveness of D5W perineural injection in SCN-E cases with a larger sample and more targeted methods.
CONCLUSION
Perineural D5W injection can be a long-term analgesic therapy in patients with SCN-E who have failed other conventional therapies.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent has been obtained from all individuals included for the publication of the case report and the accompanying images.
STANDARDS OF REPORTING
CARE guidelines were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


