All published articles of this journal are available on ScienceDirect.
Pain and Decision-Making: Interrelated Through Homeostasis
Abstract
Background:
Pain is a multidimensional experience that motivates organisms to engage in behavioral repertoire to deal with potential life-threatening situations that are a threat to homeostatic function. The aim of this mini-review was to highlight the nature of pain, the role that pain has as a motivational drive to impact higher-order cognitive processes, such as decision making, and how these processes are intimately integrated with homeostatic mechanisms.
Conclusion:
Both conceptual and neurobiological overlap suggest a close interaction of decision-making, pain, and homeostasis. Pain, decision-making and homeostasis are interconnected through a common denominator of survival and must be considered when assessing pain-related issues and treatments.
1. INTRODUCTION
Homeostasis is an ongoing auto-regulatory process in the body that maintains a relatively steady internal psychological and physiological equilibrium despite acting external forces and is exclusively for survival [1]. A stable state is required for optimal functioning of an organism and is dependent on autonomic, neuroendocrine, and behavioral regulatory mechanisms that are tightly interconnected. Each of these regulatory mechanisms buffers changes in the environment or within the body through cascading responses from each of these systems to restore imbalances [2-4]. Thus, these mechanisms are biological and unconscious in nature, working in an organized and hierarchical manner to revert to a balanced state. Yet, the homeostatic activity can also be a function of tangible behavior, motivating an organism to relieve distress and promote survival. Typical homeostatic motivational drives such as hunger and thirst are often used as classic examples. In recent years, however, the importance of ongoing auto-regulatory processes related to the motivational drive of pain is also being recognized [5-8]. Therefore, the purpose of this paper is to highlight the importance of pain as a motivational drive that directs behavior and ultimately impacts higher-order cognitive processes, such as decision making, via interrelated homeostatic processes.
1.1. Multidimensionality of Pain
Similar to other motivational drives that have a multidimensional experience, pain is often regarded as a subjective and universal phenomenon that has biological, psychological, and social implications [9-12]. The International Association for the Study of Pain defines pain as an “unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in such terms” [13]. This definition highlights the importance of not only the sensory and emotional aspects but also the potential for pain, suggesting cognitive processes associated with previous or future tissue damage. Thus, this definition presents pain not as a unimodal experience, but as a multifaceted phenomenon.
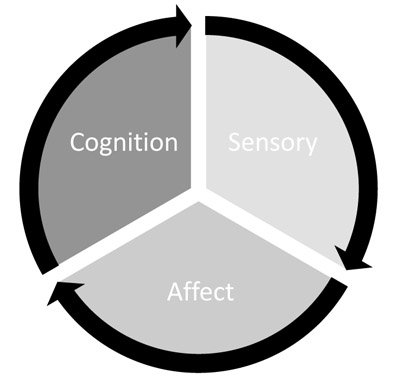
Melzack and Casey (1968) described this multidimensionality and divided pain into three components: the sensory-discriminative, the affective-motivational, and the cognitive-evaluative (Fig. 1). Through sensory neurons, the sensory-discriminative component provides information detailing the size, location, and intensity of pain, therefore encoding the physical, qualitative disruption of pain [5, 8, 10, 14-17]. The second component, affective-motivational, provides the emotional response of how unpleasant or aversive pain is perceived, along with the motivation to relieve the pain [5, 11, 18]. Somatic and autonomic reflexes and neurobiological and endocrine changes are also associated with this dimension in response to pain input. These together produce an emotional and motivational quality that drives the need for survival. The cognitive component involves the attitudes, beliefs, and expectations of pain to determine how noxious stimulus information is processed and conveyed into a response or output [19-22]. Consequently, an important aspect of cognition in pain processing is related to higher order cognitive functioning, such as decision making, and the interaction of decision-making processing during pain. Each of the components of pain provides a critical aspect of the pain experience and functioning together, produce a holistic perceptual experience of pain.
In addition to this multidimensionality perspective, pain has also been described as a homeostatic emotion [5]. Like homeostatic drives, homeostatic emotions evoke behavior in response to changes in homeostasis. This “primordial feeling” is defined by two key components, sensory and affective/motivational, that are necessary for promoting this equilibrium [23]. Pain, of course, is adaptive and motivational in nature especially when acute [12, 24, 25]. Acute pain is short in duration, self-limited, and deficient in psychosocial or biological changes disproportionate to the pain intensity [26, 27]. Due to these qualities, acute pain also contains sensory qualities that alert changes to the ideal state. While chronic pain may no longer retain this quality since this pain persists longer than the removal of the noxious stimulus or beyond tissue damage repair, chronic pain may still preserve its unpleasantness and motivation despite sensory, emotional, and behavioral alterations [27-29]. It is the affective and unpleasant qualia of pain that is the strongest contender in promoting behavior by providing a negative motivational state [5, 30]. Thus, within these qualifications, pain can be considered a driving force in homeostasis and plays an essential role in motivating an organism through emotional, attentional, and sensory mechanisms.
The importance of pain and its quality as a homeostatic emotion can be further explained through drive-reduction theory (Fig. 2) [31]. Pain creates an imbalanced state and an unpleasant affect needing to be resolved. This, in turn, motivates an organism to maintain internal stability by reacting in favor of survival. In other words, the affective-motivational component of pain is directly associated with the homeostatic and adaptive nature of pain. When pain (i.e. drive) is experienced by the organism, it disrupts homeostasis (i.e. need) and creates an unpleasant state. As a result, pain demands attention and requires a response that drives the organism to resolve, maintain, or revert to homeostasis by means of either escape or avoidance of the painful situation [5, 16, 31-35]. Therefore, we propose that pain drives homeostatic behavior through emotional/motivational processes and cognitive processes, including elements associated with decision-making.

1.2. Components of Decision-Making
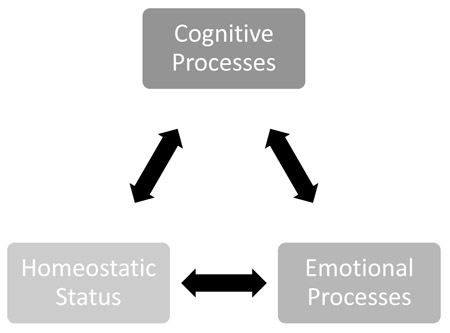
Decision-making is regarded as the cognitive process that involves identifying and assessing possible alternatives in order to solve a problem or achieve some objective [36]. Good decision-making processing is especially advantageous and is critical for survival. Under normal circumstances, decision-making involves evaluating an almost unlimited number of alternatives to a current situation to assess the best outcome of long-term behavioral consequences [37-39]. When decisions are suitable, cognition ensures that the ratio of benefits, costs, and consequences are favorable to current needs and optimizing utility at small costs [40-42]. Information regarding homeostatic status, sensory input, and prediction of future threats or benefits are required for decision processes. This is essential in ensuring the maximum number of ongoing, and possibly competing for homeostatic needs are met. Emotional or somatic processes also aid in guiding behavior and decisions. This occurs in a biased manner even in situations where there are no definitive right or wrong answers and instead cause intuitions that lead to a more perceived correct choice [43-45]. Therefore, like pain, decision-making is also influenced by cognitive and emotional processes along with homeostatic information. Such overlap strongly suggests a strong association among homeostasis, pain, and decision-making processing (Fig. 3).
1.3. Conceptual and Neurobiological Overlap
As seen above, pain and decision-making share very similar qualities. Decision-making directly reflects two of the three modalities that comprise pain - both containing aspects of emotional and cognitive processes. Pain is associated with the affective-motivational component that mirrors the emotional processing that occurs within decision-making, and general cognitive processes that are required for decision-making reflects the cognitive-evaluative component of pain. Furthermore, decision-making utilizes input from homeostatic processes, while pain disrupts homeostasis and in light of sensory and/or affective input, provides the drive needed for an organism to make decisions and react in favor of restoring homeostasis. Emotional and cognitive processes are also key players that may modulate pain experiences and behavioral outcomes. In fact, emotions and mood influence both pain and decisions where negative emotional states may increase the unpleasantness of pain even though intensity is maintained [23, 46, 47] or increase the likelihood for risky decisions [17, 48-50]. These negative states, however, do signal homeostatic imbalances that are most likely related to survival so these outcomes may not be unreasonable considering the imperfect state. To further complicate this interrelated relationship, changes in cognitive functions are also associated with pain, particularly with chronic pain and these cognitive alterations may either be a direct result of pain or pain may indirectly cause effects on cognitive function [48]. It is possible to suggest that alterations in cognitive functioning in response to chronic pain such as deficits in decision-making [48, 51], attention [30, 52, 53], and memory [54, 55] may be the result of the body’s means at coping and reflect the act of achieving equilibrium in response to constant pain. This may be driven in part to a purely emotional response, lacking in rational choice, utilization of limited resources, the interaction of neuromodulators, neuroplasticity, or a combination of all these factors [56]. Therefore, although the behavioral outcomes may not always be immediately beneficial to the individual, changes to morphology, concentration of neurotransmitters and other cellular activity are sanctioned in hopes to aid and modulate pain. Furthermore, reorganization and plasticity resulting from incoming stimuli have also been implicated in acute pain suggesting that modulation of the pain experience may be critical to survival [57, 58]. It is understood however that processes have optimal thresholds and if pain remains despite cortical changes as with chronic pain, these alternations may disrupt other homeostatic states causing more harm than good causing a positive feedback loop to address new homeostatic disturbances. Nonetheless, it should be apparent that pain and decision-making are interconnected and are greatly involved in increasing our fitness and survival through homeostatic processes especially in regard to acute pain. As one would predict, this relationship should be reflected through common neurobiological substrates, where a host of neural correlates that aid in pain processing is also involved in decision-making processes (Table 1) [59-64].
Since decision-making and pain have the common goal to engage behavior to maintain homeostasis, and imbalances of homeostasis impact decision-making and pain processes, various cortical and subcortical areas that subserve basic needs, somatic and autonomic responses, and behavioral expressions must be involved [50, 65, 66]. For example, the hypothalamus and autonomic brainstem nuclei (basal forebrain, ventral striatum, Periaqueductal Gray (PAG), and other brain-stem nuclei) are responsible for generating the corresponding somatic responses from stimuli in the presence of a decision [67]. The amygdala provides the negative or positive valence of the stimuli, promotes exploration, and demands attention towards a particular stimulus. Since the amygdala encodes the somatic valence or importance of the stimuli, it also serves as a convergence-divergence zone where the stimulus (primary inducer) or thought of the stimulus (secondary inducer) is coupled with a response [68, 69]. This processing is important to help guide future decisions since previous associations and outcomes play a major role in directing future decisions.
| Cortical and Subcortical Areas | Involvement in Pain | Involvement in Decision-Making |
|---|---|---|
| Amygdala | Affective/motivational component of pain; emotional significance; attention; pain modulation [44] | Impulsive emotional responses; emotional salience; attention; emotions for learned associations [30] |
| Anterior Cingulate Cortex (ACC) | Affective/motivational component of pain; emotional significance; pain modulation; error [18] | Cognition; motor control; motivation salience; error detection; conflict; anticipation; reward assessment [41] |
| Cerebellum | Affective/motivational and cognitive/evaluative component of pain; emotion; cognition; motor control [45] | Attention; working memory; reasoning; problem solving under uncertainty [46] |
| Hypothalamus | Sensory/discrimative, affective/motivational component of pain; relay station/ascending pain pathway; coding pain; attention [47] | Motivation; emotional salience (48) |
| Insula | Affective/motivational and sensory/discrimative component of pain; pain modulation [49] | Awareness; memory, executive functioning; association cues; motivation [40] |
| Nucleus Accumbens | Affective/motivational component of pain; emotional valence; pain modulation [50] | Learning associations; emotional salience/valence of reward or punishment; motivation [51] |
| Orbitofrontal Cortex (OFC) | Affective/motivational and cognitive/evaluative component of pain; pain valuation; cognitive [52] | Reward/pain processing; working memory; associations of emotion and stimuli [53] |
| Parabrachial Nucleus (PBN) | Affective/motivational and sensory/discrimative component of pain; arousal; target in ascending nociceptive pathway [54] | Not directly but plays a role in pleasure and pain; arousal |
| Periaqueductal Gray (PAG) | Affective/motivational and cognitive/evaluative component of pain; arousal; attention; control center of pain modulation; errors in prediction [55, 56] | Impulsive unconscious behaviors [57] |
| Prefrontal Cortex (PFC) | Affective/motivational and cognitive/evaluative component of pain; pain modulation; attention [58] | Social/moral reasoning [35]; Attention; Learning; adaptive decision-making; integration of associative information [59] Ventromedial: working-memory; impulsivity; future consquences; emotional salience of reward or punishment [60] |
| Primary Somatosensory (S1) | Sensory/discrimative and cognitive/evaluative component of pain; attention; previous experience; pain intensity [13, 61] | ? |
| Secondary Somatosensory (S2) | Sensory/discrimative and cognitive/evaluative component of pain; attention; pain valuation; pain intensity [62] | Past and present information (39) |
| Thalamus | Sensory/discrimative, affective/motivational, and cognitive/evaluative component of pain; relay station; coding pain; attention [63] | Works in conjunction with PFC for learning; adaptive decision-making; integration of associative information [59] |
Additional areas of the prefrontal cortex, including the anterior cingulate, ventromedial prefrontal, lateral and dorsolateral prefrontal, and orbitofrontal cortex are also responsible for higher processing in regards to decision-making [69]. As a whole, the prefrontal cortex is proposed to be responsible for executive functions of cognition, especially regarding making decisions and controlling attention [70, 71]. Within the prefrontal cortex, the anterior cingulate cortex is critical for detecting an error, conflict, or task difficulty. The anterior cingulate cortex is also involved in calculating benefits and costs associated with stimuli outcomes [20, 62, 72] and has been shown to play a role in processing the affective/motivational dimension of pain [21, 33, 62, 73]. Thus, the anterior cingulate cortex plays a critical role in pain affect and evaluating our decisions by assessing multiple factors.
The ventromedial prefrontal cortex also plays a role in decision-making [74, 75] and is involved in the integration of emotion and cognition. The ventromedial prefrontal cortex is responsible for the “gut feeling” potentiated by the emotion of a good or bad decision in presence of a moral dilemma [43, 44, 76]. Regardless if there are no correct answers on moral tasks, emotions ultimately provide a decision that feels more correct. Though this heuristic can enhance decisions, emotions can also reduce the efficacy of rational decisions.
The lateral prefrontal cortex is critical for executive control and, along with the anterior cingulate cortex and the parietal cortex, is partly responsible for control of attention [70]. Although the lateral prefrontal cortex and especially the dorsolateral prefrontal cortex is thought to be primarily involved in purely cognitive functioning, recent studies have revealed evidence for the integration of cognition and emotion in this area [77, 78]. In fact, emotional valence photos could modulate activity in these areas where pleasant photos increased activity and unpleasant photos decreased activity compared to control photos [79].
The orbitofrontal cortex, like the amygdala, can discriminate positive and negative values of stimuli by integrating sensory and affective information. This stimulus evaluation provided by the amygdala and the orbitofrontal cortex appears to also be responsible for action strategies for current and also future anticipatory occurrences [53, 80-82]. As a result, both regions refer to critical associations with previous decisions and consequences such that current decisions will be better suited to the wanted outcome.
2. DISCUSSION
Homeostasis, pain, and cognition can individually impact behavior through powerful biological mechanisms. However, these systems do not act in isolation. Indeed, there is considerable conceptual and neurobiological overlap that highlights the close interaction of decision-making, pain, and homeostasis. Reductively, this relationship is founded upon pain requiring and eliciting a decision. Certainly, when pain is presented, an organism must ultimately choose whether to escape/avoid or approach/allow it. Yet this interplay becomes more profound when the rationale for similarity is extended further to include the shared characteristics of homeostasis and emotional and cognitive qualities. Ultimately then, the decision to either escape or allow pain entirely depends on the current homeostatic information which includes current affective, attentional, motivational influences. Under this idea, it is no wonder that pain is a multidimensional phenomenon that directly impacts homeostasis through its three modalities. To summarize entirely, the sensory component of pain allows an organism to be aware of where and how the homeostatic disturbance occurs, while the affective-motivational component demands attention and motivates an organism to resolve the pain, and the cognitive-evaluative component provides the organism with a solution- a behavioral choice based on previous and current pain situations and what outcomes that are associated with those choices. Thus, pain disturbs the ideal state homeostasis requires and as a result, drives a decision. This positive feedback loop is necessary for survival.
Even more, under this same notion, decision-making is considered the aiding force of homeostasis. Decisions utilize homeostatic information to cognitively and emotionally assess which alternatives provide a more ideal state determined by cost versus benefit. As a result, decisions promote homeostasis and aid in reverting to the ideal state. Pain, in this case, is a stressor and changes the ideal state, whereas the decision to escape or avoid pain returns homeostatic equilibrium. Additionally, because homeostasis can be reduced to a mechanism of maintenance, resistance, and survival, pain and decision-making are necessary for completing that loop [1, 5, 32, 83]. This then could suggest that these entities may not be truly separate systems, but rather interconnected.
Evolutionarily, this concept makes sense. By design, each of these biological mechanisms plays a role in increasing the odds of survival. Functionally, it is anatomically and behaviorally efficient to combine similar like mechanisms along parallel, if not overlapping biological pathways especially when each component revolves around a central goal of survival. Thus, the link between homeostasis, pain, and cognition can be further expounded through the perspective of survival through three means: an alerting system through pain, a mechanism of checks and balances provided by homeostasis, and requirement of action driven by decision-making.
CONCLUSION
The undeniable overlap in both neural signatures and a conceptual understanding of these phenomenon suggests the importance of survival. However, the numerous factors that can individually influence pain, decisions, and homeostasis, may also play a role in modulating survival. Thus, it may be imperative to consider this complex and integrated relationship when attempting to understand the multi-dimensionality of pain and may provide further insight into how pain and pain treatments may differ across individuals.
FUNDING
No funding sources were provided.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.