RESEARCH ARTICLE
Comparison of Effects of Two Drugs (Pregabalin & Celecoxib) on 24 hours Post-Operative Pain Intensity in Patients Undergoing Tibia Fracture Surgery
Amir Sobhani Eraghi1, Iman Azizpour1, *, Mikaiel Hajializade1
Article Information
Identifiers and Pagination:
Year: 2021Volume: 14
First Page: 14
Last Page: 21
Publisher ID: TOPAINJ-14-14
DOI: 10.2174/1876386302114010014
Article History:
Received Date: 14/2/2021Revision Received Date: 27/4/2021
Acceptance Date: 3/6/2021
Electronic publication date: 10/11/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Celecoxib is widely used in post-operative cases because of its ability to reduce postoperative opioid drug use. Currently, the use of this drug is common in post-operative cases. In various studies, pregabalin was used for the management of pain after spinal surgery to reduce the need for opioids.
Objectives:
Since the treatment of tibia fractures and surgery is painful and has a long-term recovery, this study aimed to compare the effect of two drugs (pregabalin and celecoxib) on pain severity at 24 h postoperatively in patients having tibia fracture surgery. This would mark significant progress in taking the proper drug.
Methods:
In this probability clinical experiment, the sample consisted of 50 patients scheduled for tibia fractures, who were selected from the table of random numbers. Then, the patients were assigned into two groups: celecoxib (Group C) and pregabalin (Group P). In the first group, celecoxib was administered to patients at 1 h pre-operatively at a dose of 200 mg and 1 h post-operatively at a dose of 200 mg. In the second treatment group, patients received pregabalin at 1 h pre-operatively at a dose of 200 mg and 1 h post-operatively at a dose of 200 mg orally. Then VAS (visual analog scale) scores were recorded at 6, 12, and 24 h after surgery. Finally, using SPSS software, qualitative variables were compared according to their percentage by the Chi-square test. For quantitative analysis of variables, the mean value of each group was calculated. The comparison of means was made by t-test.
Results:
The VAS score was considerably lower at 24 hours after surgery in the pregabalin group than in the celecoxib-treated group. However, after 6 and 12 h of surgery, no statistically meaningful difference was observed. A less analgesic effect was observed in the group treated with celecoxib than pregabalin, which was statistically significant.
Conclusion:
Pregabalin improves postoperative pain, and it has more analgesic effects than celecoxib.
1. INTRODUCTION
Currently, the literature abounds with controversies regarding tibial fracture treatment [1]. These fractures are common, and their treatment brings numerous challenges. According to NCHS, 490,000 people suffer from tibial fractures in the United States each year [2]. The yearly rate of tibial fractures is 1 per two thousand persons [3]. The anterior-medial face of the tibia below the skin is the underlying reason for its susceptibility to fractures. Tibia is the bone frequently affected by open fractures, mainly due to the vulnerability of tibial zone skin and connective tissue [4]. A tibia fracture is fairly common in young adults, and its adverse effects include disability and ensuing economic damages for the patients. On a larger scale, this disease can wreak havoc on the health system of countries.
Surgical stimulation causes central sensitization and leads to irritability in the surgical site, and increases postoperative pain.
Central inhibition in the nervous system by analgesics can achieve short-term advantages, including post-surgery pain reduction and accelerated recovery. On the other hand, its long-term effects include improved quality of life and diminished chronic pains [5]. To reduce pain during surgery, preemptive analgesia is applied by central desensitisation in the place of surgical incision [6].
In patients, numbness and pain-alleviating effects can mitigate stress reactions, including water and sodium retention, hyper-metabolism, tachycardia, hypertension, and wound healing latency [7-10]. Besides, deep sedation can lead to adverse effects such as the increased risk of pneumonia, vascular thrombosis, and hypotension [11-13].
Celecoxib is one of the drugs in the COX-II inhibitor group that has fewer cardiovascular complications than other drugs in this group [14]. Some studies have also shown the effectiveness of celecoxib in reducing pain after various surgeries and the need for opioids [15, 16].
Pregabalin is a newly constructed molecule from GABA and a ligand for gamma 2 alpha that has anaesthetic, anti-epileptic, anti-stress, and sleep modulating effects. Pregabalin controls acute pains after surgery by inhibiting the Spinothalamic pathway [17]. Besides, in most patients experiencing stress, pregabalin could be effective [18]. Accumulating evidence suggests that pregabalin can be useful for toothache after spine surgeries and after laparoscopic cholecystectomy [19-22].
A study by Ittichaikulthol et al. on pregabalin effects on decreasing the requirement for morphine after hysterectomy showed that 300 mg pregabalin just one hour before hysterectomy decreases morphine requirement effectively. Thus, it could be an alternative drug besides morphine [23].
Clarke et al. conducted a study on pregabalin and gabapentin to prevent chronic pain after surgery and indicated that they could reduce pain. According to their findings, the use of gabapentin for 2 months after surgery could decrease chronic pains effectively. Besides, 3 clinical trials have reported reduced chronic pain after the surgery in patients receiving gabapentin [24].
A review study by Dauri et al. indicated that taking gabapentin and pregabalin in comparison to placebo and opioids reduced pain. However, it was not as successful and effective as diet therapy. In their study, 37 cases were compared with the goal of decreasing acute pain after surgery [25].
Akhavan Akbari et al. explored the impact of oral administration of pregabalin on post-surgery pain in patients having lower extremity surgery. The results showed that a single oral dose of preoperative 150 mg was effective in mitigating postoperative pain. It reduces the need for operation and decreases pethidine dosage administered in orthopedic surgeries. Furthermore, the VAS score declined in the treatment group compared to the group receiving placebo. Nevertheless, postoperative vomiting and nausea, sedation levels at 2 h and 6 h postoperatively, as well as pethidine intake were significantly decreased in the pregabalin group [26].
This study aims to draw a comparison between the effect of two drugs, pregabalin and celecoxib, on postoperative pain in patients having tibia fracture surgery after 24 h. This marks a step forward in selecting the proper drug. Since tibial fracture surgery is particularly challenging for patients and it takes a long time for patients to have clinical recovery and improvement, administering the proper drug (pregabalin or celecoxib) 24 h after tibial fracture surgery can help mitigate pain and enhance their quality of life.
Due to a lack of studies on the effect of pregabalin and celecoxib on reducing patient's pain after surgery and on the comparison of these drugs, we decided to compare the effect of these two drugs on reducing pain after surgery.
It is expected that there is a difference between the effectiveness of these two drugs in reducing postoperative pain.
According to the research hypothesis, there is a difference between the effectiveness of pregabalin and celecoxib on pain within 24 hours after surgery in patients with tibial fractures.
2. METHODS
2.1. Study Design and Drug Groups
This is a randomized clinical trial, and the sample consisted of 50 patients having elective tibial fracture surgery who were selected from the table of random numbers. The subjects were split into two groups: Group receiving celecoxib (Group C) and the group receiving pregabalin (Group P). They had surgery according to the scheduled date. For the purpose of randomization, patients undergoing surgery on odd days were assigned to the pregabalin group, and those undergoing an operation on even days were allocated to the celecoxib group. Inclusion criteria were 18 and 50 years of age and ASA 1-2 physical status with tibial fractures. Exclusion criteria were more than 50 or less than 18 years of age, overweight (BMI> 20%), history of allergy to celecoxib or pregabalin, alcohol abuse or substance dependence, medical conditions such as hypertension, asthma, and diabetes, kidney and liver dysfunction, a history of chronic pains, using pain killers at 6 h before surgery, and surgery lasting more than 3 h. Celecoxib was taken at 1 h pre-operatively (a dose of 200 mg) and 1 h post-operatively (a dose of 200 mg) in Group C. The other group received pregabalin at 1 hour pre-operatively (a dose of 200 mg) and 1 h post-operatively (a dose of 200 mg) orally. The assay of variables was conducted at 6, 12, and 24 h post-operatively. For this purpose, a checklist based on postoperative nausea and vomiting (PONV), VAS score, blood pressure, heart rate, sedation score, length of post-operative use of painkillers, and the total amount of pain killers taken by patients. The checklists were completed by the researcher. The data analysis was conducted by SPSS 22. The qualitative variation was determined by measuring the percentage of amplitude and comparing it with the CHI square. Moreover, quantitative variables were identified using t-test and mean score. The case-related data recorded in checklists will be published based on the results.
As reported by Prasad et al., the mean pain score in the groups receiving celecoxib and pregabalin post-operatively was 3, 58± 0, 98 and 4,55 ± 1,03, respectively, with CI= 0,05 and the test power of 90%. A sample size of n=50 (25 in each group) was calculated. Written informed consent was obtained from individuals. Information on all individuals was kept by the researchers. The study was approved by the Ethics Committee of Iran University of Medical Sciences with code: IR.IUMS.REC.1394.8921215050. The study imposed no financial burden on the patients.
2.2. Statistical Methods
The quantitative analysis was based on mean ± standard deviation and qualitative variability based on percentages. For data with normal distribution, quantitative and qualitative variables were compared by t-test. For abnormally distributed data, the comparison was conducted by the Mann-Whitney U test. The qualitative variables were compared by Chi-Square or Fischer test. We used Pearson correlation and Spearman rank correlation coefficient to investigate quantitative variables. To evaluate the discrepancy of study indices in patients and the presence of certain features in patients as confounders, we used multivariate logistic regression analysis and reported the results as odds ratio (95% CI). The data analysis was performed by SPSS 22 and SAS 9.1. A significance level less than 0.05 was considered.
The research was performed in compliance with the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of Iran University of Medical Sciences with an ethical approval number of IR.IUMS.REC.1394.8921215050. Written informed consent was attained from the participants prior to the study. The participants were ensured about the confidentiality of information. The study had no financial burden on the patients.
3. RESULTS
The sample consisted of 83 patients aged 34 - 50 years with a history of tibial fractures scheduled for elective surgery. Total 19 patients who did not meet inclusion criteria were removed after initial screening. Also, 8 patients were excluded based on exclusion criteria, and 6 refused to participate in the study. In the end, n=50 (60.2%) patients were included in the study and randomized into two groups of n=25 each.
Participants in the two groups were compared in terms of demographics and basic clinical characteristics (age, weight, height, duration of surgery, pulse rate, mean arterial pressure). The mean age of patients was 32.3 years (SD =4.3) in Group C and 32.6 years (SD=6.4) in Group P, but no significant difference was observed between the two groups in this regard. Moreover, the average weight was 55.47 ± 5.6 kg in Group C and 54.35±6.83 kg in Group P, and there were no significant weight differences between the two groups. The average height was 164.07±3.43 cm in Group C and 164.48 ± 4.45 cm in Group P, but no significant difference was found between the two groups. None of the patients had a history of medical conditions, including hypertension, diabetes, etc. The mean length of surgery was 102±10.2 min in Group C and 108±14.8 min in Group P, but the two groups were not significantly different. The average pulse rate in Group C was 95.66 ± 11.2, and it was 92.17±13.9 in Group P. Moreover, the average mean arterial pressure in Group C was 66.27±13.4, and it was 65.1±13.26 in Group P, and the two groups were not significantly different (Table 1). The hospitalization condition was similar for all patients.
| - | Group C (treated with celecoxib) | Group P (treated with Pregabalin) | P- value |
| Age mean (SD) | 32.3 years (SD: 4.3) | 32.6 years (SD=6.4) | 0.3 |
| Weight mean (Standard deviation) | 55.47 ± 5.6 | 54.35±6.83 | 0.54 |
| Height mean (SD) | 164.07±3.43 | 164.48 ± 4.45 | 0.33 |
| Surgery duration mean(SD) | 102±10.2 min | 108±14.8 min | 0.83 |
| PR mean(SD) | 95.66 ± 11.2 | 92.17±13.9 | 0.22 |
| MAP mean(SD) | 66.27±13.4 | 65.1±13.26 | 0.5 |
The average pain severity was recorded. The VAS score was recorded at 6 h (6.35±0.31), 12 h (7.02 ±0.32), and 24 h (7.26±0.36) after surgery in Group P. Also, VAS score at 6 h (7.14± 0.22), 12 h (7.19±0.37), and 24 h (7.27±0.4) after surgery was calculated in Group C. The mean pain severity in Group P was lower than that of Group C after 6 h, but the difference was not statistically significant (p = 0.13). Furthermore, the mean pain severity was significantly lower in Group P than in Group C after 12 h (p = 0.25). Finally, the mean pain severity after 24 h was lower in Group P than in Group C, and the difference was significant (p = 0.02) (Table 2).
| - | Group C | Group P | P-value |
|---|---|---|---|
| Mean pain score 6 hours after the operation | 7.14±0.22 | 6.35±0.31 | 0.13 |
| Mean pain score 12 hours after the operation | 7.19±0.37 | 7.02±0.32 | 0.25 |
| Mean pain score 24 hours after the operation | 7.27±0.4 | 7.35±0.1 | 0.02 |
In general, irrespective of the time factor, pain severity in Group C was significantly higher than Group P (p = 0.04).
Group C received 362 doses of narcotics, and Group P received 346 doses of narcotics during the treatment. The first-time requirement for narcotics was 242±4.9 min in Group C, and 251.2±4.8 min in Group P. The two groups were not significantly different (p = 0.12).
The number of Group C patients who had received 1 dose of opioids at 6-12-24 h postoperatively was significantly lower than that of Group P (p = 0.37). Also, the number of Group P patients who had received 2 doses of opioids at 6-12-24-h postoperatively was significantly lower than that of Group C (p = 0.13). Similarly, the number of Group C patients who had received over 2 doses of opioids at 6-12-24 h postoperatively was significantly lower than that of Group P (P = 0.04).
In general, the pain relief medication given to patients in Group C was significantly greater than that of patients in Group P (p = 0.02) (Table 3).
In both groups, Ramsay sedation score (RSS) was less than 2 (Table 4).
The repeated measures ANOVA showed that all subscales improved significantly in the two groups (VAS: F = 45.2, p = 0.000, RSS: F = 149.99, p = 0,000, heart rate: F = 26.834, p = 0.000, MAP: F = 32.00, p = 0.000, F = 6.846, p = 0.004) (Table 5). The analysis of time-treatment interaction suggested that celecoxib had a greater effect than pregabalin over time, as depicted in subscales of RSS (P = 0.002) and VAS (P =0.000) (Table 5).
No significant difference was observed between the two groups in other cases. In Group C (treated with celecoxib), the mean changes in RSS score after treatment were significantly different than that of Group P (treated with pregabalin) and had a large effect size of 0.94 (mean difference (MD), 95% CI = 3.23 (1.22-5.26) p = 0.002). However, the two groups were not significantly different in the mean scores of the RSS subscale at 12 h after surgery (P = 0.21). The VAS score of therapeutic efficacy of celecoxib/pregabalin was significantly increased at 12 and 24 h, with the midpoint (MD, 95% CI =1.6(0.8-3.1) and endpoint (MD, 95% CI =4.8(2.43-7.45). However, the therapeutic effect was greater at 24 h (D = 0.94) and 12 h after surgery (D = 0.53), while exhibiting significance (p = 0.051) for a higher effect (D= 0.51), celecoxib versus pregabalin was present in the number of drug users at the end of the post-operative 24 h. (MD, 95% CI =0.72(000-1.43)). The comparison of response rates reflected a significant difference between the two groups (p = 0.002) RSS (p = 0.001) in terms of VAS and the number of patients using the drug (p = 0.051) at 24 h postoperatively (Table 6).
| Group | 6 hours after the operation | 12 hours after the operation | 24 hours after the operation | P-value | |
|---|---|---|---|---|---|
| 1 dose | C | 19 (76%) | 13 (52%) | 6(24%) | 0.37 |
| P | 22 (88%) | 21(84%) | 3(12%) | ||
| 2 dose | C | 6 (24%) | 12(48%) | 19(76%) | 0.13 |
| P | 3(12%) | 4(16%) | 19(76%) | ||
| More than 2 | C | - | - | 1(4%) | 0.04 |
| P | - | - | 3(12%) | ||
| Sum | C | 25(100%) | 25(100%) | 25(100%) | 0.02 |
| P | 25(100%) | 25(100%) | 25(100%) |
Table 4. Comparison of RSS in Groups C and P.
| - | Group C | Group P | P-value |
|---|---|---|---|
| RSS 6 hours after operation | RSS1: 15(60%) RSS2: 10 (40%) |
RSS1: 9 (36%) RSS2: 16 (64%) |
0.4 |
| RSS 12 hours after operation | RSS1: 12 (48%) RSS2: 13 (52%) |
RSS1: 12 (48%) RSS2: 13 (52%) |
0.07 |
| RSS 24 hours after operation | RSS1: 9 (36%) RSS2: 16 (64%) |
RSS1: 13 (52%) RSS2: 12 (48%) |
0.04 |
| Efficiency | Time | Reaction against time | In patients | |||
| F | P | F | P | F | P | |
| RSS: | ||||||
| Celecoxib (C) | 149.992 | 0 | 5.568 | 0.002 | 0.176 | 0.677 |
| Pregabalin (P) | ||||||
| Heart Rate: | ||||||
| Celecoxib (C) | 26.834 | 00 | 0.2 | 0. 5 | 0.8 | 0.076 |
| Pregabalin (P) | ||||||
| Blood Pressure: | ||||||
| Celecoxib (C) | 32.004 | 00 | 2.317 | 0.094 | 0.008 | 0.92 |
| Pregabalin (P) | ||||||
| VAS: | ||||||
| Celecoxib (C) | 45.2 | 00 | 10.005 | 000 | 000 | 0.9 |
| Pregabalin (P) | ||||||
| Number of people in need of opiate after surgery: | ||||||
| Celecoxib (C) | 6.846 | 0.004 | 3.002 | 0.069 | 0.06 | 0.76 |
| Pregabalin (P) | ||||||
| Efficiency | Mean Difference (CI%95) | T (df) | P | Cohen’s D (95%CI) | |
|---|---|---|---|---|---|
| RSS: | |||||
| 12 hours after operation | 1.32(0.74-3.38) | 1.39(45.47) | 0.21 | 0.33(0.18-0.83) | |
| 24 hours after operation | 3.23(1.22-5.26) | 3.69(60) | 0.002 | 0.94(0.41-1.46) | |
| Heart rate: | |||||
| 12 hours after operation | 0.9(0.73-2.53) | 1.11(60) | 0.272 | 0.28(0.22-0.78) | |
| 24 hours after operation | 0.13(2.03_2.48) | 0,12(60) | 0.905 | 0.03(0.47-0.53) | |
| Blood pressure: | |||||
| 12 hours after operation | 0.97(0.08-2.02) | 1.84(60) | 0.07 | 0.47(0.04-0.97) | |
| 24 hours after operation | 1.26(0.21-2.73) | 1.77(55.21) | 0.093 | 0.43(0.07-0.94) | |
| VAS: | |||||
| 12 hours after operation | 1.6(0.8-3.1) | 2.1(60) | 0.04 | 0.53(0.02-1.04) | |
| 24 hours after operation | 4.8(2.43-7.45) | 3.68(60) | 0.001 | 0.94(0.41-1.46) | |
| Number of people in need of analgesic: | |||||
| 12 hours after operation | 0.39(0.05-0.83) | 1.79(39.13) | 0.081 | 0.45(0.06-0.96) | |
| 24 hours after operation | 0.72(000-1.43) | 1.99(60) | 0.051 | 0.51(0.00-1.01) | |
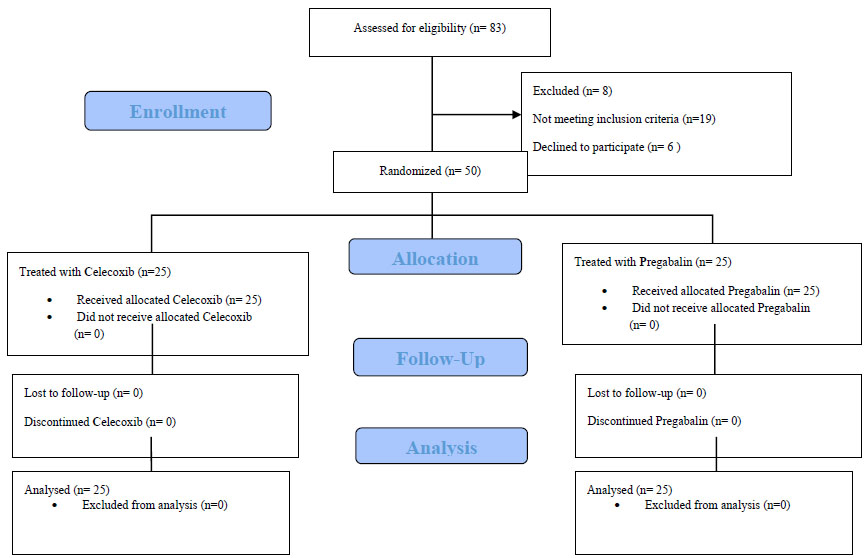 |
Fig. (1). CONSORT flow diagram. |
4. DISCUSSION
Patients often complain of post-surgery pain. Pain signals activate a cycle of messages in the body's somatosensory system and enhance the stimulation of pain [3].
Celecoxib, as a COX-II inhibitor, induces anesthesia at spinal and supraspinal levels. The absorption of oral celecoxib is fairly complete, and the highest plasma level is reached after 6 to 9 h. Given its fat solubility feature, it can cross the blood-brain barrier. As a GABA analogue drug, pregabalin inhibits the secretion of several pain-related neurotransmitters. With a half-life of 5.5 to 6.5, it is independent of dose and repetition [5].
We used 200 mg of celecoxib and pregabalin at 1 h pre-operatively and measured variables at 24 h post-operatively. RSS and VAS, at 12 and 24 h after surgery, revealed considerable inter-group differences. Moreover, RSS was considerably higher in celecoxib-treated subjects than in the pregabalin group, which may suggest the greater analgesic effect of celecoxib compared to pregabalin.
Similar to our study, Montazeri and Ghobadian reported that celecoxib increased the length of spinal anesthesia [27]. Singh, Ota, and Liu administered 100 to 200 mg of celecoxib at 1-1.5 h in patients before spinal anesthesia and found that it had a significant effect on prolonging anesthesia [28].
Baidya et al. observed that in patients treated with pregabalin, the need for post-operative analgesia was decreased [29].
In the present study, the two groups were significantly different in the mean length of anesthesia and VAS score. Moreover, in patients treated with pregabalin, the length of pain relief was significantly shorter at 12 and 24 h after surgery, which may have a diminishing effect on postoperative pain of patients.
Ittichaikulthol et al. explored the impact of pregabalin on abdominal pain and morphine intake after surgery in patients undergoing hysterectomy. They reported that 300 mg of pregabalin at 1 h ahead of hysterectomy had a significant effect on reducing morphine consumption. The study also proposed pregabalin as a substitution of morphine for postoperative analgesia [23].
A review study by Clarke et al. on preventing chronic postoperative pain using gabapentin and pregabalin shows that the use of pregabalin and gabapentin relieves chronic postoperative pain. Of 474 papers reviewed in this study, 11 were discussed in the aforementioned article. This study advises that gabapentin use reduces the incidence of chronic postoperative pain by up to 2 months postoperatively. In 3 articles studied, a significant difference was observed in the incidence of chronic postoperative pain in patients receiving gabapentin [24].
In the same vein, Dauri et al. reported a significant reduction in the intake of pregabalin and gabapentin and pain in comparison to the placebo group. It is while other treatments have been barely effective. The primary goal was the treatment of acute postoperative pain with pregabalin and gabapentin, which had been discussed in 37 articles [25].
Akhavanakbari et al. carried out a trial to assess the impact of oral pregabalin on postoperative pain in patients undergoing lower extremity surgery. They observed that a single oral dose of 150 mg pregabalin preoperatively led to a decline in pethidine usage during orthopedic surgeries. They reported a lower VAS score throughout the study in comparison to the placebo group. Nonetheless, postoperative vomiting and nausea declined at 2 h and 6 h after surgery, and pethidine intake dropped in the pregabalin group compared to the placebo group [26].
A study by Prasad et al. aimed to compare the administration of preoperative oral celecoxib and preoperative pain and postoperative pain under spinal anesthesia, comparing oral pregabalin (150 mg) with oral celecoxib (200 mg) after spinal anesthesia; it prolongs the rate of postoperative pain relief and is less sedative. This study was performed on 90 women undergoing vaginal hysterectomy, and the VAS score was significantly lower in the pregabalin group than in the celecoxib group. Postoperative opioid use and VAS scores in both the pregabalin and celecoxib groups were lower [30].
The study of Mammoto et al. aimed at the effect of celecoxib on pain management after knee replacement surgery; they concluded that celecoxib treatment reduced postoperative pain and VAS [31].
According to the mentioned studies, both celecoxib and pregabalin are effective in reducing the amount of pain after various surgeries, and we concluded in this study that the effect of pregabalin is more effective than celecoxib in reducing pain, especially after tibia fracture surgery.
5. LIMITATIONS
Some patients did not agree to participate in the study initially, but after explaining to them the study aims and procedure, they agreed to participate in the study.
6. SUGGESTIONS
The assessment of the efficacy of celecoxib vs. pregabalin in the alleviation of postoperative pain in the present research can drastically change the method of postoperative pain reduction. We recommend the use of pregabalin due to its greater effects in relieving pain in patients with orthopedic fractures after surgery. This study could also serve as a reference for future studies to address the following questions: Can pregabalin improve the therapeutic impacts of celecoxib? Is it possible to use pregabalin in order to alleviate the celecoxib’s side effects in patients?
ETHICAL STATEMENT
The study was approved by the ethics committee of Iran University of Medical Sciences, Iran with an ethical approval number of IR.IUMS.REC.1394.8921215050. The registration number was not necessarily required by the institution for conducting this study.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Inform consent was obtained from all patients included in the study.
STANDARD OF REPORTING
CONSORT guidelines were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
FUNDING
This study was financially supported by the Iran University of Medical Sciences.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the Rasool Akram Medical Complex Clinical Research Development Center (RCRDC) for its technical and editorial assists.