Hindquarter Amputation as a Successful Treatment of Chronic Pain in an Adolescent with PTEN Hamartoma Tumor Syndrome
Abstract
Background:
The PTEN hamartoma tumor syndrome comprises a group of rare conditions caused by germline mutations of the tumor suppressor gene PTEN (Phosphatase and TENsin homolog deleted on chromosome 10). They include Cowden syndrome, Lhermitte-Duclos disease, and Bannayan–Riley–Ruvalcaba syndrome, but it appears likely that these conditions represent the spectrum of PTEN hamartoma tumor syndrome, in which penetrance and clinical variability play significant roles. The clinical features of PTEN hamartoma tumor syndrome vary between individuals and can appear at any age. They include an increased risk for certain types of cancers and benign tumors, as well as tumor-like malformations (hamartomas) and neurodevelopmental disorders.
Case Presentation:
We report the case of an adolescent male with PTEN hamartoma tumor syndrome who developed severe chronic pain in the context of multiple arteriovenous malformations of the left lower limb. He successfully underwent hindquarter amputation to manage a nonhealing ulcer, recurrent episodes of life-threatening hemorrhage, and escalating analgesic requirements. He had a remarkable recovery with neither pain nor phantom sensory issues and significant improvement in his quality of life.
Conclusion:
Despite chronic preoperative pain, the radical surgery in this patient with PTEN hamartoma tumor syndrome resulted in an improved quality of life and virtually no long-term pain or analgesia requirements.
1. BACKGROUND
The PTEN hamartoma tumor syndrome (PHTS) (MIM 158350) comprises a group of rare conditions caused by germline mutations of the tumor suppressor gene PTEN (Phosphatase and TENsin homolog deleted on chromosome 10) [1, 2]. They include Cowden syndrome, Lhermitte-Duclos disease, and Bannayan–Riley–Ruvalcaba syndrome [3, 4], but it appears likely that these conditions represent the spectrum of PHTS, in which penetrance and clinical variability play significant roles [5, 6].
Hindquarter amputation is an extreme but recognized treatment of pain, with significant mortality (1.5%), marked morbidity, and a 35-45% wound infection rate. Around 60% of individuals require analgesia for phantom symptoms [7]. Here, we present an adolescent with PHTS with associated lower limb vascular anomalies and intractable pain, bleeding, and ulceration who was successfully treated with a hindquarter amputation.
2. CASE PRESENTATION
Parental consent and later patient consent were obtained prior to providing the following case report and accompanying images. The patient has been de-identified.
A 12-year-old boy was admitted to the hospital with a nonhealing wound on his left lower leg. He identified as an indigenous Australian and lived in a remote community more than 500 km from our institution (the closest pediatric tertiary referral center). His past medical history was significant for the excision of an epidermal nevus on his left foot at age 6, the release of left foot contracture at age 7, deep venous thrombosis of the left leg at age 9, significant mobility issues and obesity (91kg, BMI 36). He had marked macrocephaly (Occipital-Frontal Circumference 61 cm, >2 SD above the mean) and lower limb asymmetry (Fig. 1). Multiple vascular malformations of the left lower limb, in conjunction with the history of an epidermal nevus, led to the presumptive diagnosis of CLOVES syndrome (Congenital Lipomatous Overgrowth, Vascular Malformations, Epidermal Nevi, Spinal/Skeletal Anomalies/Scoliosis syndrome). A sample of skin from the affected area was taken, and a heterozygous PTEN nonsense variant was identified (PTEN:c.445C>T (pQ149*) in exon 5. This variant was subsequently also identified in blood, confirming germline PHTS. Consequently, the diagnosis was revised to PTEN hamartoma tumor syndrome. The variant was also identified in his father, who has macrocephaly, a history of leukemia, and a right frontal meningioma. However, he does not have vascular malformations.
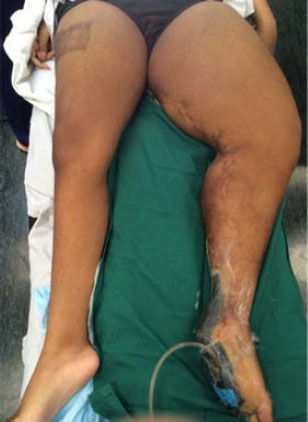
Six months prior to admission, a left Achilles tendon lengthening procedure with ankle and subtalar joint release was performed to treat a severe equinus deformity. This surgery was complicated by a nonhealing ulcer. He received wound care and three split skin grafts, which were unsuccessful in resolving the problem. The pain was managed with amitriptyline 10mg nocte and simple analgesia as required. The individual was discharged back to his local hospital for daily dressing changes with an expectation of resolution. Eleven months after his initial surgery, he was readmitted to the tertiary hospital for the management of the same nonhealing wound.
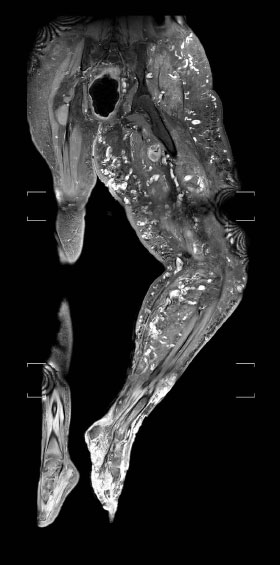
During this prolonged admission, the management involved (at minimum) weekly visits to the operating room for wound debridement and dressing changes. The individual also underwent 13 sessions of hyperbaric therapy, which was discontinued due to his refusal to participate. Sirolimus was trialed after international consultation, but as the arteriovenous malformations continued to increase in size, it ceased. Despite all efforts, the necrotic wounds relentlessly extended from his foot and ankle to involve his lower leg and thigh (Fig. 2). Spontaneous hemorrhage developed from the leg wound, requiring emergent surgical exploration and blood transfusion on four occasions during an eight-week period. Angiography demonstrated worsening diffuse arteriovenous malformation involving virtually all left pelvis, left upper, and left lower leg. MRI Coronal T2 sequences of the lower limbs demonstrated disproportionate tissue overgrowth and multiple disorganised dilated vasculatures throughout the left lower limb (Fig. 3).
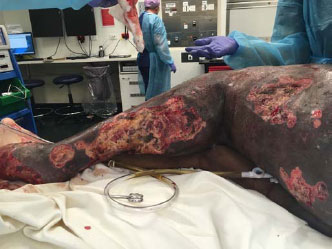
On this admission to the hospital, the individual’s only regular analgesic was amitriptyline 10mg nocte, and acetaminophen and oxycodone as required. During his long admission, many other opioid and non-opioid analgesics were trialed. The most effective combination of analgesic medications included methadone, tramadol, fentanyl IV (as patient-controlled analgesia) clonidine, pregabalin, lidocaine patches and ketamine. Other opioids used at times (including during periods of opioid rotation) were hydromorphone, oxycodone, and morphine. Daily opioid analgesic consumption increased throughout his stay in the hospital. Despite significant multimodal analgesia, he was often screaming in pain for prolonged periods, particularly following surgical procedures and dressing changes. He often slept for many hours during the day, stating that he had slept poorly overnight due to pain. He routinely exhibited poor self-care, frequently refused to leave his room, and had minimal engagement with multidisciplinary teams.
Twenty-one months after his original tendon lengthening surgery, hindquarter amputation was discussed with the individual and his family. Throughout the individual’s admission, regular psychological, psychiatric and social work input was evaluated. A thorough psychological evaluation of the likelihood of coping with amputation was difficult due to a lack of engagement. Despite this, the individual and family were adamant about attempting amputation, as they felt that his current situation was not compatible with a meaningful quality of life or indeed, survival.
The perceived surgical risks were life-threatening bleeding due to known extensive vascular abnormalities, wound infection and sepsis, severe postoperative pain issues, and expected poor cooperation with rehabilitation. Despite these concerns, a decision was made to proceed to left hindquarter amputation with a free flap latissimus dorsi reconstruction, as this represented the greatest chance for survival. The individual was aged 13 years and 10 months at the time.
The surgery was preceded by the placement of multiple occluding coils in the left internal iliac artery and a 14mm Atlas™ balloon dilation catheter in the aorta, which could be inflated in the event of catastrophic bleeding. Simultaneously, an IVC filter was placed to prevent pulmonary embolus. Hindquarter amputation took 17.5 hours, after which the individual returned to the Pediatric Intensive Care Unit. He then had a planned return to the theatre three days later for a free flap latissimus dorsi reconstruction. Following the reconstruction, he remained intubated and sedated and was extubated eight days later. Recovery was complicated by a profuse gastrointestinal bleed, which was found on endoscopy to be caused by two large bleeding gastric ulcers. This was managed successfully with omeprazole.
Pre-operatively specific consideration was made for the prevention of phantom limb pain (PLP), particularly given the individual’s recognized risk factor of chronic pre-amputation pain [8]. He had been treated with amitriptyline, but it was ceased months prior. At the time of amputation surgery, he had been taking gabapentin for 10 months, lidocaine patches for 7 months and a ketamine infusion continuously for 5 months, varying from 8 to 16mg/hour. It was therefore felt that his pre-emptive analgesic prevention of PLP had been optimized. A single-shot sciatic nerve block was performed at the end of the surgery, but no catheter was placed due to the plan for prolonged post-operative intubation.
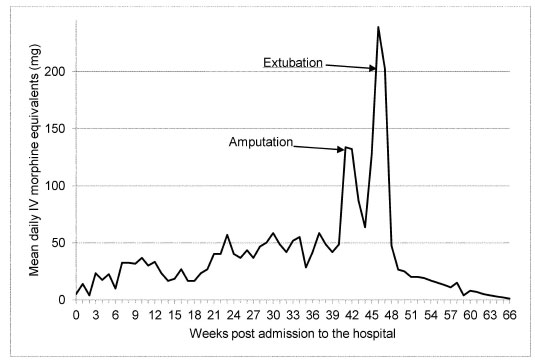
Post-amputation opioid analgesic requirements in 24-hour intravenous (IV) morphine equivalents (Fig. 4) rapidly declined to 1mg IV morphine equivalent at the time of discharge, 21 weeks postoperatively. One week prior to amputation, maximal patient-reported pain scores ranged from 3 to 10 (weekly average 8). In the third postoperative week (once extubated), he reported maximal pain scores ranging from 0 to 5 (weekly average 2).
Following hindquarter amputation, the individual’s mood rapidly improved. He enjoyed sitting up and appeared to be motivated to make progress with rehabilitation. He engaged in treatment plans with medical and allied health staff, ventured out of his room, and participated in self-care activities. He continued to have intermittent periods of unwillingness to engage, but these were markedly less frequent than prior to the amputation. He had regular psychiatric and social worker follow-ups with specific mention of increased engagement and motivation.
The individual was discharged home with community occupational therapy and physical therapy 66 weeks after admission, which was two years and one month after his initial elective surgery. He was transferred to his wheelchair independently and was keen to play wheelchair-based sports and engage in outings, such as concerts. He reported no chronic or phantom limb pain and was on no regular medication. He regularly followed up with the rehabilitation team to assess physical function and psychological well-being, which continued for three years post-discharge.
3. DISCUSSION
Hindquarter amputation is an uncommonly performed operation due to significant morbidity and mortality. Here, we reported a case of a remarkable outcome of the resolution of severe chronic pain, improved mood, self-care, and engagement in treatment following hindquarter amputation in a child with PHTS.
The decision to perform hindquarter amputation in a young individual without malignant disease was not taken lightly. There were concerns regarding the significant risk of phantom limb pain as well as the expectation of poor cooperation with rehabilitation. Despite a prolonged period of inpatient treatment, there was inexorable worsening in ulceration, bleeding, pain, and distress, and it was agreed by a large multidisciplinary team that the surgery represented the best opportunity to improve his quality of life.
Our individual had one serious complication postoperatively, with a life-threatening gastrointestinal bleed, but the long-term outcome was excellent. Prior to amputation, he was also experiencing repeated bleeding requiring blood transfusion and urgent surgery. In preoperative counselling, it was difficult to estimate the potential physical and psychological risks and benefits of this surgery. Available data are generally related to adults with malignant disease and usually combined with radical limb amputation surgery of different types. Some patients included in the reported series had also undergone preoperative radiotherapy and/or chemotherapy [9, 10]. The most relevant studies reported postoperative complications in 43-44% of patients (12-20% flap necrosis, 16-37% wound infection) [9, 10]. Although postoperative mortality for this surgery in the past was high [11], more recently, well-resourced tertiary referral centers, such as ours, have reported mortality rates of 0-7% for non-malignant disease [12, 13].
Following the surgery, our individual had a rapid and marked improvement in his pain, mood, and mobility. One retrospective study examined the emotional and psychological effects of limb amputation in 12 children with metastatic cancer and a limited lifespan, six of which had surgery comparable to our patient [14]. Of the 11 patients who were wheelchair-bound or bedridden prior to surgery, mobility subsequently improved after the surgery in eight patients. It was also found that overall pain scores were lower one week and three months after the surgery compared to immediately prior to surgery. A study of 76 young adults with hip disarticulation or similar surgery following traumatic injury found that all patients were mobile in long-term follow-up, with only 9.3% using a wheelchair [15]. Mental health and social functioning scores were similar to the general population [15].
Our individual has so far not reported phantom limb pain (PLP), but this was a chief concern of the treating team prior to amputation. Prevention of PLP with preemptive analgesia is controversial but includes the utilization of tricyclic antidepressants, anticonvulsants, sodium channel blockers and NMDA receptor antagonists, as well as regional anaesthetic techniques [8]. In a study on young adults undergoing traumatic amputation at this level, 90.8% of patients reported long-term PLP [15]. Other studies on older adults have reported PLP in 73-100% of long-term survivors of this type of surgery [9, 10, 16].
Reports of this type of surgery in childhood for any indication other than sarcoma are few [17, 18]. There are, however, two previous similar case reports of young patients with PHTS undergoing lower limb amputation. The first patient was a 7-year-old boy who had heart failure and difficulty walking due to severe deformities in his right leg. He underwent right leg amputation with hip disarticulation. That individual also had a de novo germline PTEN:c.1003C>T (p.R335X) base transversion in exon 8, and the tumor had a second somatic mutation (p.R130X) in exon 5 [19]. The second patient was a 10-year-old girl with Bannayan–Riley–Ruvalcaba syndrome [20] with an extensive arteriovenous malformation extending from the right buttock to the lower limb accompanied by muscle atrophy. Genetic testing in that individual revealed a de novo nonsense PTEN variant c.1003C>T (R335X) in exon 8, resulting in a premature termination codon [20]. Although a “second hit” in PTEN was not identified in our individual, it is hypothesized that his severe leg asymmetry/capillary malformation is due to a second nonidentified somatic PTEN variant in the affected tissues.
CONCLUSION
In this paper, we described an adolescent boy with PHTS who underwent hindquarter amputation to treat a severe lower limb vascular malformation with ulceration and bleeding. Despite chronic preoperative pain, the radical surgery resulted in an improved quality of life and virtually no long-term pain or analgesia requirement. With such a rare disease, reporting this excellent outcome adds to the sparse literature on the condition.
LIST OF ABBREVIATIONS
| PHTS | = PTEN Hamartoma Tumor Syndrome |
| PLP | = Phantom Limb Pain |
| IV | = Intravenous |
AUTHORS’ CONTRIBUTIONS
NW, FT, and LB were involved in obtaining the clinical information as well as drafting and approving the manuscript. LF, CB, and PY contributed clinical information and discussion relevant to their specialties and edited and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Written consent for publication of this case report was provided by the parent of the individual at the time of hospitalization. Subsequently, written consent was also gained from the patient after they reached 18 years of age. Consent specifically included the use of clinical photographs.
STANDARDS OF REPORTING
CARE guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank Silvana De Rosa for administrative support and Rachel Dineen for data collection.


