All published articles of this journal are available on ScienceDirect.
Ligamentous Knee Joint Instability: Association with Chronic Conditions of the Knee and Treatment with Prolotherapy
Abstract
Ligamentous knee joint instability and other conditions associated with knee dysfunction are common musculoskeletal complaints that affect a large percentage of the global population. A healthy knee has normal joint mechanics and can maintain its stability as it responds to the forces placed upon it. Once undue forces, whether from injury, wear and tear, or overuse, cause the soft tissue structures of the knee to stretch beyond their normal range of motion, they can become lax, elongated, damaged, or torn, especially the ligaments. This condition, known as ligamentous knee instability, causes destructive joint forces to occur, which results in the development of other pathophysiologic conditions related to knee dysfunction, including osteoarthritis, patellar pain syndromes, tendinopathies, meniscus tears, and osteochondral defects. Traditional treatments address the consequences of joint instability, such as synovitis and joint swelling, but do not address the underlying ligament and/or disease that led to the joint instability. Prolotherapy promotes the repair of injured or degenerated tissues, such as ligaments, tendons, and menisci, by stimulating the physiological healing process of the joint. This process corrects the underlying joint instability, reduces associated pain, improves knee function, and has the potential to slow the degenerative process.
1. INTRODUCTION
The knee is the largest and one of the most easily injured joints in the body. It is not surprising that knee pain develops, often becoming a chronic and disabling condition that can have a severe and widespread impact not only on affected patients and their caretakers but also on their healthcare resources.
Knee pain can develop because of injury, disease, or wear and tear from overuse. As of 2014, according to the data available to date, 1 in every 5 American adults experienced chronic knee pain, an increase of 2.3% over 2012 figures [ 1]. This increase includes nearly 1 in 10 persons aged 18 to 44, fully 1 in 4 persons aged 45 to 64, and 1 in 3 persons aged 65 and older. As the population ages, the prevalence of knee pain will continue to increase; this is especially true for those in the 45 to 64 age cohort experiencing joint problems earlier than in previous years [ 1, 2]. Total and partial knee arthroplasties (knee replacement surgeries) are common treatments for end-stage osteoarthritis (OA) in the knee joint, and the incidence of knee arthroplasties has risen in the last decade. According to findings from the National Inpatient Sample conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ), the number of total joint arthroplasties in the United States is anticipated to rise to approximately 935,000 by 2030 [ 3]. In a report from the United States Bone and Joint Initiative, knee joint arthroplasties are one of the most common surgeries performed every year and are ranked 4 th in the number of orthopedic procedures performed (joint/soft tissue injections and arthrocentesis are 1-3) [ 1]. Furthermore, a population-based study examining 40 years of medical records of patients who underwent primary total hip arthroplasties and total knee arthroplasties indicates that patients with total knee replacements have a 30-45% chance of requiring a surgical procedure in a contralateral cognate joint [ 4].
The aim of this paper is to provide a better understanding of the knee by describing its anatomy and physiology and its risk for and response to injury and disease as they relate to joint instability and chronic pain, which has led to the high prevalence of knee arthroscopy and revision surgery. We will also discuss the advantages and disadvantages of conventional treatment modalities while focusing on a lesser-known but more effective and safer treatment option, i.e., prolotherapy, a regenerative injection therapy (RIT) that promotes the healing process by stimulating cell proliferation in injured tissues.
2. KNEE JOINT STABILITY
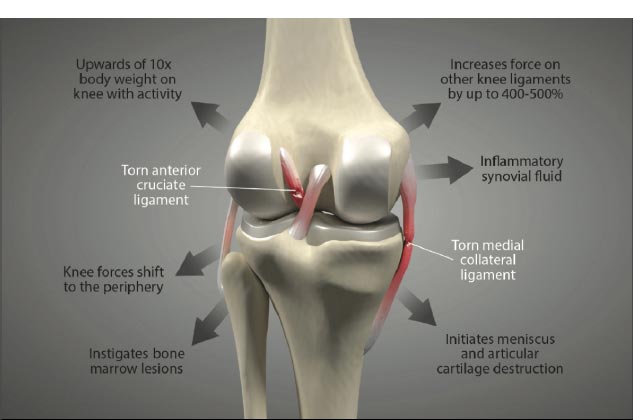
Knee stability depends on the stacked anatomical configuration of the femur and tibia and the static forces provided by the soft tissue structures of the joint, such as the menisci, joint capsule, muscles, and ligaments, which are the main stabilizers of the joint (Fig. 1 and Table 1). When these structures are damaged, either by acute or chronic injury, the knee joint becomes lax and unstable. Joint laxity and instability shift the centrally located primary load-bearing area to a more peripheral location, causing the force to overload the subchondral bone and articular cartilage in that area [ 5] (Fig. 2) [ 6, 7]. Damage to the knee joint occurs because the structures, including the articular cartilage, meniscus, and subchondral bone, are not conditioned to handle this sudden shift in the location of load-bearing onto the periphery of the joint.
Structures that stabilize the knee joint are mentioned in Table 1.
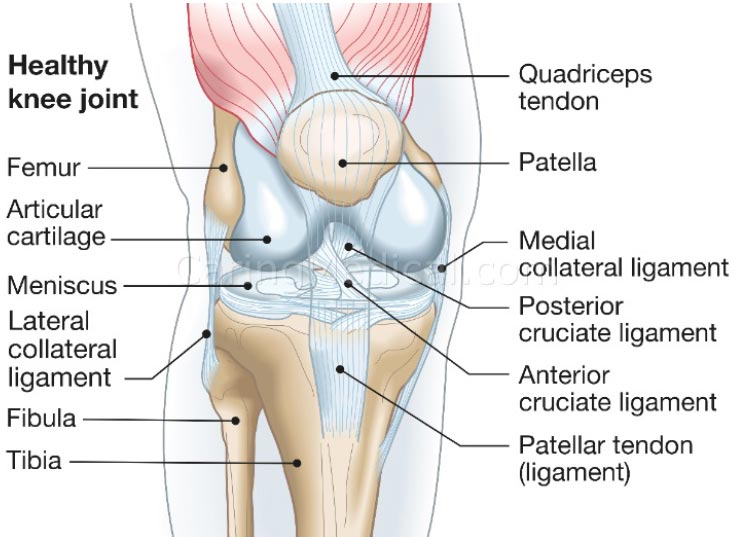
| Tibiofemoral Joint | Stabilizing Action |
|---|---|
| Anterior cruciate ligament (ACL) | Resists anterolateral displacement of the tibia on the femur, especially at 90° of flexion; resists varus displacement at 0° of flexion. |
| Articular cartilage | Allows gliding movement, shock absorption, and translation/distribution of forces. |
| Femur and tibia | Provide static stability during standing when the femur is positioned posteriorly to the tibia. |
| Iliotibial band | Works with quadriceps to provide stability to the lateral knee during repetitive motion (running, cycling, walking, etc.). |
| Joint capsule | Encloses the tibiofemoral (and patellofemoral) joints to increase stability. |
| Lateral collateral ligament (LCL) | Resists varus displacement at 30° of flexion; resists posterolateral rotatory displacement with flexion that is less than approximately 50°. |
| Medial collateral ligament (MCL) | Resists valgus angulation; works in concert with ACL to provide restraint to axial rotation. |
| Menisci | Deepen articular surface between femur and tibia; equalize load distribution and shock absorption. |
| Posterior cruciate ligament (PCL) | Resists posterior tibial displacement, especially at 90° of flexion; resists varus displacement at 0° of flexion. |
| Patellofemoral Joint | Stabilizing Action |
| Hamstring muscles | Stabilize the knee as it moves into flexion. |
| Joint capsule | Encloses the patellofemoral (and tibiofemoral) joints to increase stability. |
| Lateral femoral condyle | Limits lateral motion during flexion. |
| Quadriceps muscles | Stabilize the knee as it moves into extension. |
| Quadriceps tendon and patellar ligament | Stabilize the patella as the leg moves into flexion and extension. |
| Reticular tissue | Predominates in locations with high cellular content; statically stabilizes the patella. |
3. LIGAMENTS OF THE KNEE
In addition to the capsule and menisci, the stability, strength, and movement of the knee depend on 4 ligaments: 2 intracapsular ligaments (ACL and PCL) and 2 extracapsular ligaments (MCL and LCL). These ligaments are the most important passive stabilizers of the joint. They maintain smooth joint motion and guide the movement of the femur and tibia as they move through flexion and extension. They play a critical role in guiding the kinematics of movement and preventing excessive joint motion, which can damage the articular cartilage, menisci, and other soft tissue structures [ 8].
The extracapsular collateral ligaments of the knee provide stability to the medial and lateral sides of the joint. The medial collateral ligament (MCL) is a strong, flat band that extends from the medial condyle of the femur to the medial condyle of the tibia, with some deep fibers attaching to the medial meniscus. It provides medial stability against valgus stress, forces that would push the knee joint inward (knock-kneed stance). The lateral collateral ligament (LCL) is a rounded cord that runs from the lateral condyle of the femur to the head of the fibula. It provides lateral restraint against varus stress, forces that would push the knee joint outward (bow-legged stance). Both the MCL and LCL also prevent anterior translation of the tibia on the femur, prevent lateral rotation of the tibia on the femur, and work with the anterior cruciate ligament to provide restraint to axial rotation. Any ligament injury of the knee can lead to one of the many types of instabilities in the knee (Fig. 3).
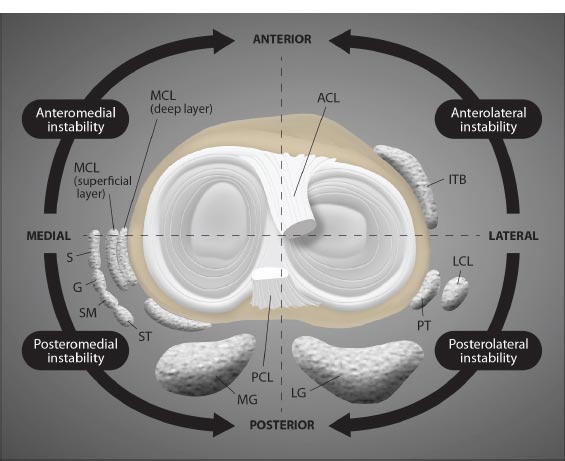
Abbreviations: ACL = anterior cruciate ligament; ITB = iliotibial band; LCL = lateral collateral ligament; PT = popliteal tendon; LG = lateral gastrocnemius; PCL = posterior cruciate ligament; MG medial gastrocnemius; ST = semitendinosus; SM = semimembranosus; G = gracilis; S = sartorius; MCL = medial collateral ligament.
Modified from Magee DJ. Orthopedic Physical Assessment. 6th Edition. St Louis, 2014, Elsevier Saunders, p. 811.
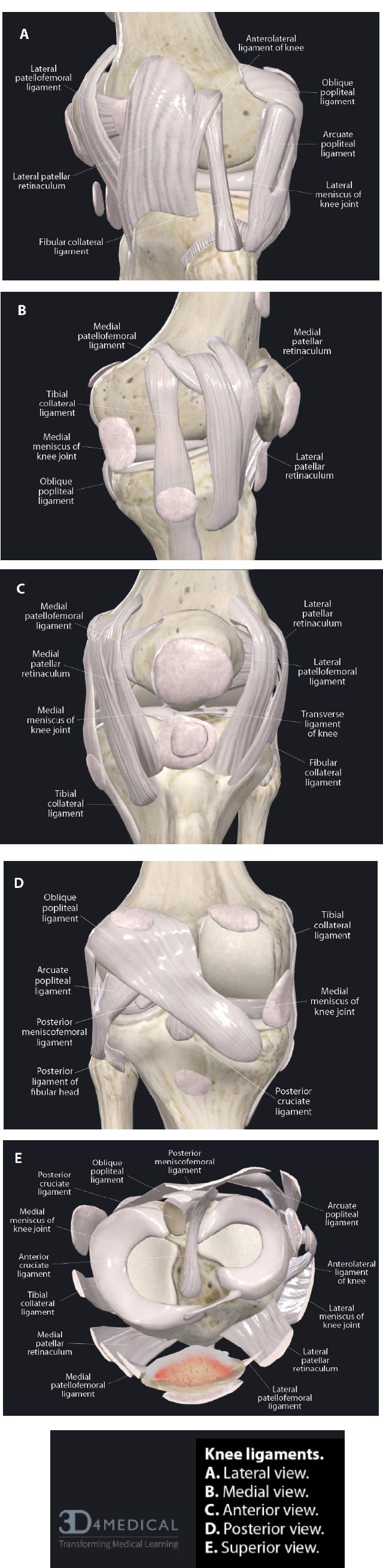
( A). Lateral view. ( B). Medial view. ( C). Anterior view. ( D). Posterior view. ( E). Superior view.
The anterior cruciate ligament (ACL) and the posterior cruciate ligament (PCL) form a crisscross in the center of the joint. The ACL runs from the anterior to the intercondylar eminence of the tibia to the posterior medial surface of the lateral condyle of the femur. The PCL runs from just posterior to the intercondylar eminence of the tibia to the anterior portion of the medial surface of the medial condyle of the femur (Fig. 4). The ACL and PCL are different from ligaments of other joints because they restrict normal, rather than abnormal, motion. The ACL is one of the most important contributors to the knee’s stability. It provides 85% of the restraint against the anterior translational force of the tibia relative to the femur when the knee is flexed 90°. It also acts as a secondary restraint to both internal and external rotations in the non-weight-bearing knee. Along with the resistance of the posterior muscles crossing the knee joint, the ACL also prevents the healthy knee from hyperextending. The PCL is the strongest ligament in the knee, as it has anterior and posterior fibers that differ in orientation. The PCL provides 95% of the restraint to posterior translation of the tibia on the femur during 90° flexion, prevents hyperextension, and limits rotation of the tibia. Together, the ACL and PCL are secondary stabilizers and provide some resistance to varus stress, which helps provide medial/lateral stability if the primary lateral structures have been disrupted. They also have a role to play in stabilizing the knee during rotation.
A fifth structure that contributes to knee stability is the quadriceps tendon. The quadriceps tendon includes the patellar tendon, which passes over the front of the knee and attaches to the patella. At the site of attachment, the patellar tendon continues as the patellar ligament, a strong, flat ligament that attaches the apex of the patella to the tibial tuberosity. The medial and lateral portions of the quadriceps tendon line, on either side of the patella, are inserted into the upper part of the tibia and joined with the joint capsule. The patellar ligament directs the force of the quadriceps femoris muscles to the tibia during the extension of the knee to stabilize the patellofemoral joint (Fig. 5).
4. THE EFFECT OF COMPRESSIVE AND SHEAR FORCES ON THE KNEE
Forces placed on the knee joint during normal activities are of considerable clinical significance. In the course of a normal year, the knee undergoes between 1-2 million motions, and the force applied to the joint during each motion is several times the body weight [ 9]. The knee joint accepts both shear and compressive forces when loaded and mobilized during action, such as sit-to-stand motions, stair climbing, and squatting. To function well, the knee joint must optimize 2 opposing requirements: stability and flexibility. Biomechanically, the healthy knee maintains a balance between stability and flexibility as it switches between weight-bearing support and functional movement during walking, running, sitting, kicking, and other similar movements that require the knee to bend [ 10]. Weight-bearing support requires joint stability, while the flexibility of the joint requires that it has a degree of laxity, which is necessary in order for the knee to bend, rotate, and then return to correct alignment in response to internal and external forces during movement [ 11].
| Activity | Force | % Body Weight | Pounds of Force |
|---|---|---|---|
| Walking | 850 N | 1/2 x BW | 100 lbs. |
| Cycling | 850 N | 1/2 x BW | 100 lbs. |
| Stair ascend | 1,500 N | 3.3 x BW | 660 lbs. |
| Stair descend | 4,000 N | 5 x BW | 1,000 lbs. |
| Jogging | 5,000 N | 7 x BW | 1,400 lbs. |
| Squatting | 5,000 N | 7 x BW | 1,400 lbs. |
| Deep squatting | 15,000 N | 20 x BW | 4,000 lbs. |
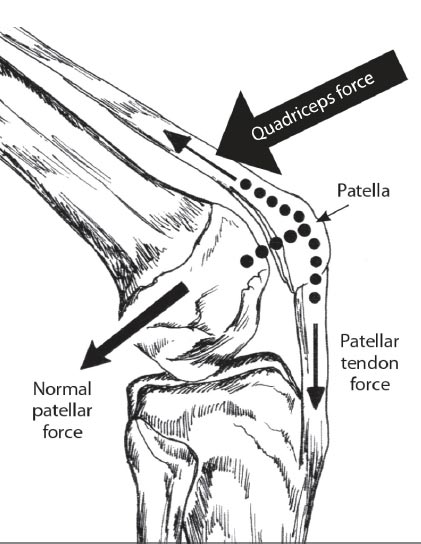
As the knee is flexed, the contact point on the patella migrates inferior to superior pole.
Adapted from: Magee D. Orthopedic Physical Assessment. 6th Edition. St. Louis, MO: Saunders; 2014. Redrawn from Neumann CV. Kinesiology of the Musculoskeletal System: Foundations for Physical Rehabilitation. St. Louis, 2002, Mosby, p.448.
4.1. Application of Force on the Healthy Knee
In the healthy knee, compressive forces are absorbed by soft tissue structures, which help to stabilize the articulation of the femur and tibia. During flexion, the top of the patella is pulled by the quadriceps tendon, while the bottom of the bone is simultaneously pulled by the patellar tendon, causing the patella to put compressive force on the posterior condyles of the femur. As the surface area of the patella is small in relation to the force applied to it, the compressive force on the patellofemoral joint is very high. At maximum flexion, the compressive force across the patellofemoral joint can be very high [ 9] (Table 2).
To ensure the femur remains on the tibial plateau, the compressive force needs to be strong enough to counter the force of the femur as it glides and rolls anteriorly while the knee flexes. As flexion increases, the compressive force that the quadriceps and patellar tendons apply to the patella becomes greater, preventing displacement of the femur (Fig. 6). As the compressive force of the patella increases, contact between the patella and femoral condyles also increases. This helps to spread the compressive force by transmitting some of it to the femur [ 9].
Modified from: Heijink A, et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012 Mar; 20(3): 423-435. Table 2.
Compressive force, or load of gravity, is also essential for the nourishment and healing of the articular cartilage since it has no blood supply. When compressive force is applied, synovial fluid is pushed into the articular cartilage for nourishment, and once relieved, fluid goes out of the articular cartilage. Physiologic loading, including dynamic compression during motion, also causes an increase in anabolic growth factors and cytokines that stimulate the building of the extracellular matrix and aid in the health and maintenance of articular cartilage, menisci, and other joint structures. When mild injury occurs, these same forces induce an anabolic healing reaction to assist the body in repair.
4.2. Forces Applied to the Misaligned Knee
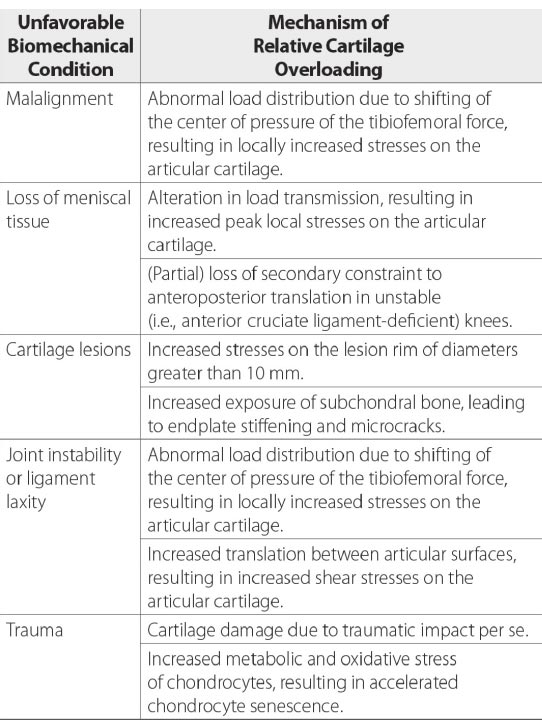
Compressive joint forces stabilize the knee as long as the tibia and fibula are in proper alignment. If the articular surfaces are out of alignment, the center of force shifts to the periphery of the joint. This contact stress is then distributed into the thin cartilage of the condyle. The change in the site of contact alters the distribution, direction, and magnitude of load applied to the joint [ 12]. Such changes in the biomechanics of the joint complex result in increased gliding of the articular surfaces of the femur and tibia on one another, causing excessive motion about the joint.
When the kinematics between the tibia and femur are changed by misalignment, the integrity of the ligaments is disrupted, and the muscles’ ability to stabilize the joint is decreased, resulting in joint instability. This provides an unfavorable biomechanical condition around the knee joint. (Fig. 7). To accommodate the strain of force, healthy ligaments elongate slightly. If the force exceeds the strength of the ligament, permanent ligament damage can occur, with resultant instability [ 12]. The ACL, for example, receives 75% of the anterior force on the knee at full knee extension and an additional 10% at up to 90 o of knee flexion [ 13]. The ACL is normally 40 mm in length. Only 2 mm separate ACL injury and total rupture. Microscopic injury to the ligament occurs at approximately 5 mm of stretch and total rupture at 7 mm (Fig. 8). Sharp, cutting-type movements (such as those done when playing soccer or football) apply sudden rotational force, which can strain the ACL up to 8 mm (20%) and cause multi-planar knee laxity, which alters the joint biomechanics [ 14, 15].
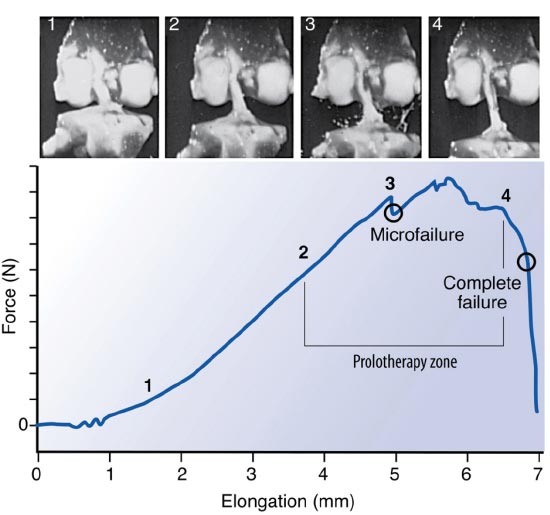
Used with permission from: Nordin M, et al. Basic Biomechanics of the Musculoskeletal System. 4th Edition. Lippincott Williams & Wilkins. Baltimore, MD. 2012.
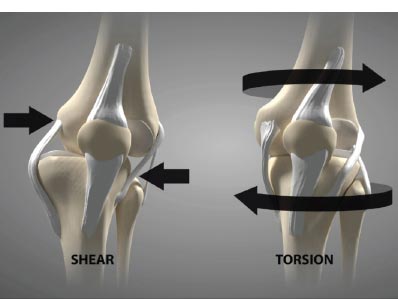
Shear force, which is negligible in the healthy knee joint (only 1-2%), is harmful in the unstable joint and increases as ligaments become laxer. For example, knees with a deficient ACL will experience 3 times as much force in the MCL and increased shear force on the tibia [ 16]. In addition to mechanical changes, shear force causes biomolecular alterations in the joint. They increase destructive inflammatory mediators, including nitric oxide, interleukin-1, tumor necrosis factor-a, metalloproteinases, and excessive reactive oxygen species (oxidation) in the synovial fluid [ 17, 18]. These inflammatory substances cause pain and swelling in the joint, and the long-term net effect of these mediators is the breakdown of the articular cartilage extracellular matrix if the excessive forces from joint instability are not resolved [ 19-21] (Fig. 9). Passive and static biomechanics that were previously well managed by the joint before the ligaments were injured now produce destructive shear forces.
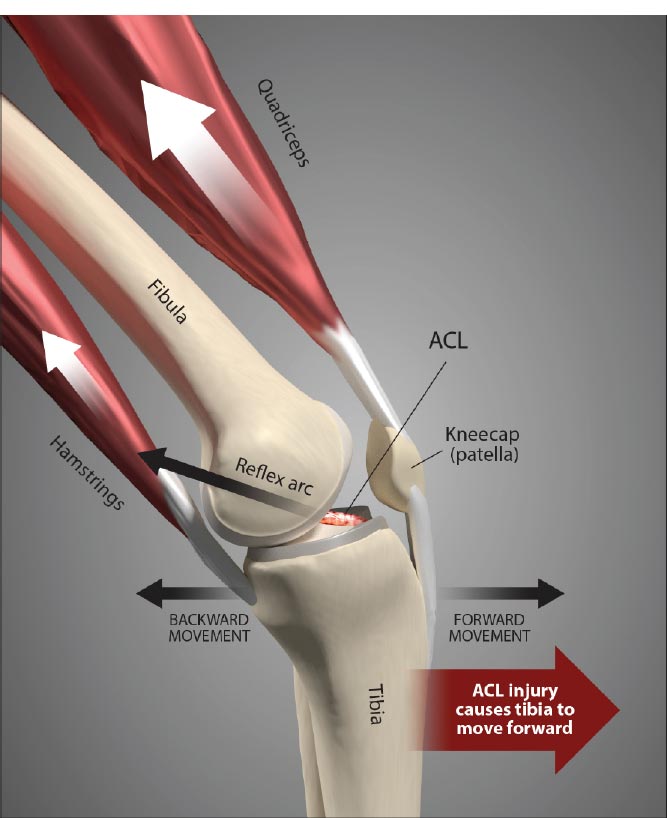
Ligament injury causes destructive forces upon the knee. It is not just the magnitude of the force that damages the joint structures when the ligaments are injured but also the location and direction of the force as well [ 19, 22]. When the femur shifts backward, and the tibia shifts forward, the patella gets pulled away from the tibia. Less lubrication leads to an increase in shear strain on the articular cartilage [ 17, 22]. High tension or shear at the edges of the joint contact regions predisposes the cartilage to splitting, tears, or fibrillation over time [ 23]. When ligaments are injured, unaligned shear forces push one part of the joint cartilage in one direction while another part of the articular cartilage is pushed in the opposite direction (Fig. 10).Continued movement, in this case, will induce pathologic loading. Excessive joint motion leads to synovitis and the production of excessive inflammatory fluid, which, if left unchecked, causes articular cartilage breakdown, dysregulated bone remodeling, and other characteristics of osteoarthritis [ 24] (Fig. 11).
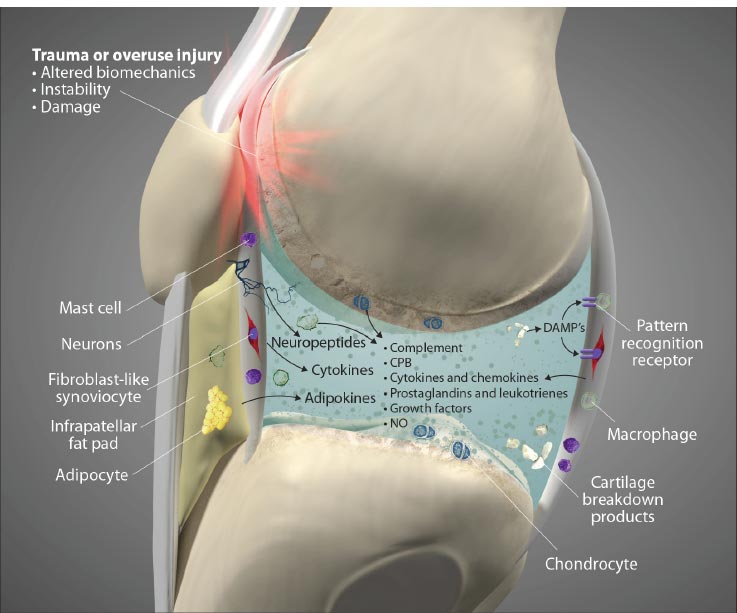
Adipocyte image modified from: https://pt.wikipedia.org/wiki/Ficheiro:Yellow_adiposetissue_in_paraffin_section_-_lipids_washed_out.jpg. Macrophage image modified from: https://commons.wikimedia.org/wiki/File:Macrophage.jpg
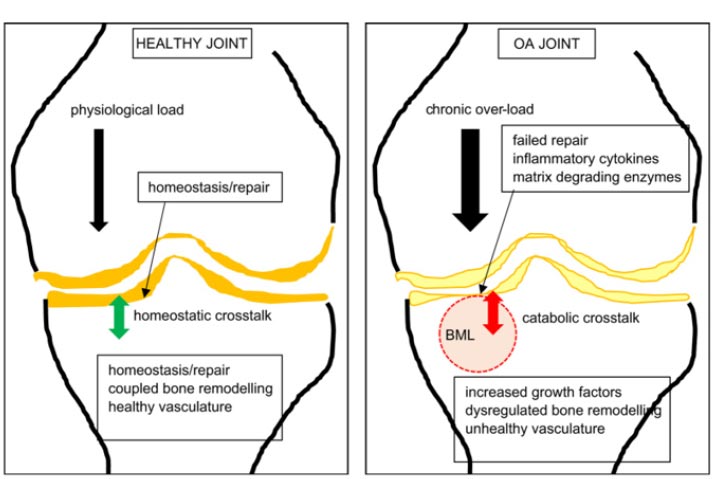
Findlay DM, Kuliwaba JS. Bone-cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 2016;4:16028. Published 2016 Sep 20. doi: 10.1038/boneres.2016.28. Copyright © 2016 The Author(s). http://creativecommons.org/licenses/by/4.0/
5. JOINT STABILITY AND INSTABILITY
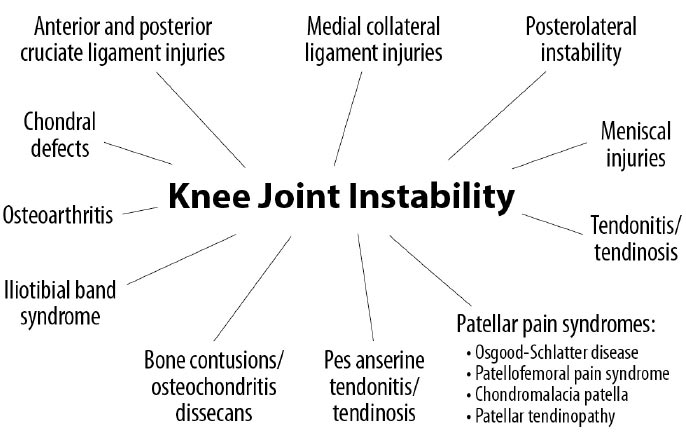
The stability of the knee joint is contingent on healthy knee structures, including the ligaments, tendons, meniscus, joint capsule, cartilage, and bony surfaces [ 25]. When an injury occurs to any of these structures, whether through athletic, traumatic, or overuse injuries, instability will result. Laxity from instability changes the contact between the bony surfaces. When this occurs, motion and applied loads substantially increase the force per unit area on specific regions of the knee, resulting in progressive degeneration of the structures [ 26, 27]. Instability can cause or contribute to many knee conditions, including osteoarthritis [ 28], tendonitis/tendinosis, chondral defects, and meniscal injuries (Fig. 12).
Joint stability is the ability of a joint to remain in, or promptly return to, proper alignment through an equalization of forces, maintained by the coordinated interplay of the soft tissue, dynamic muscle forces, and external joint load acting on or across the knee joint [ 29]. Knee stability is dependent on ligament tension and strength, congruent joint surfaces and menisci, and normal function of the musculotendinous structures [ 30]. The effectiveness of muscle contribution to functional joint stability depends on free nerve endings within the ligament, triggering a nociceptive pain impulse called the ligamento-muscular reflex to inhibit movements, which may induce stretch or injury to the ligaments and other joint structures [ 31, 32]. The most common reflex arc in the knee is the contraction of the quadriceps and hamstring muscles when there is an injury to the ACL (Fig. 13). The sensorimotor system plays an essential role in knee joint stability by maintaining a complementary relationship between (a) static, including the osseous configuration, menisci, ligaments, tendons, and capsule, and (b) dynamic restraints, which are provided by the muscles surrounding the knee joint [ 33].
With any impairment of the static and dynamic restraints, joint instability can result, thereby increasing force and pressure on the joint. Chronic joint instabilities from ligament and meniscal injuries increase knee joint loading because they significantly increase intra-articular pressures. In addition, they also decrease the articular cartilage contact area.
The ACL anatomy has important characteristics, as it is the most important ligament stabilizer in the knee (Fig. 14). Instability due to an ACL injury will precipitate the eventual breakdown of proper knee function, rotatory instability, meniscal tears, degenerated cartilage, and osteoarthritis. The disruption that occurs from changes in the normal motion and mechanics of the knee joint due to joint instability further leads to, or is associated with, the development of a host of secondary pathophysiologic conditions of the knee. Injury to one knee structure frequently affects other elements of the knee, causing ligament ruptures and meniscal tears, which often occur in combination [ 34]. If the concurrent soft tissue injuries are left untreated, the cycle of instability progresses, resulting in significant impairment [ 35, 36]. The deficit that occurs after ligament injury is persistent, with the properties of the ligament substance remaining markedly inferior both biologically and biomechanically to normal ligaments. Even after years of remodeling, they continue to exhibit abnormalities in their collagen fibrils, matrix, and structural properties [ 8]. This is further illustrated by the association between ACL injuries and meniscal, ligamentous, cartilage, and bone injuries, and osteoarthritis [ 37, 38] (Fig. 15).
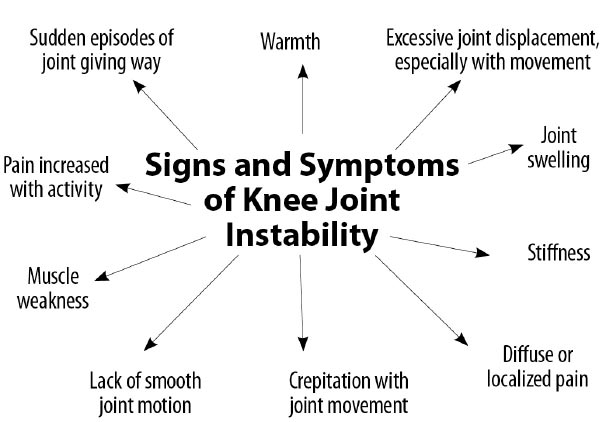
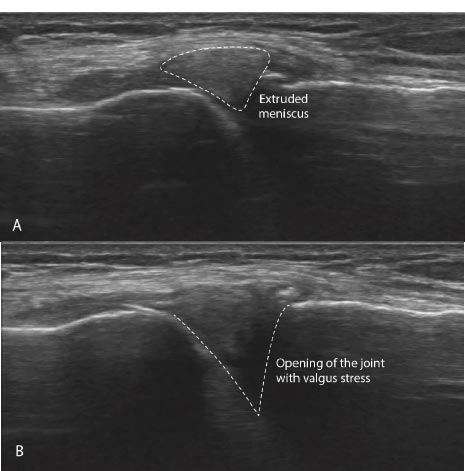
Signs and symptoms of joint instability can include pain, joint swelling, excessive joint displacement (especially with movement), crepitation with joint movement, lack of smooth joint motion, muscular weakness, and sudden episodes of a joint giving way [ 39] (Fig. 16). Most knee pain occurs with motion, but standard radiographs and MRIs are static. Diagnostic tests that demonstrate knee instability include stress X-rays, motion X-rays (fluoroscopy), and stress/motion ultrasound, where the joint is visualized functionally while in motion (Fig. 17).

1. Adamcyzk G. ACL deficient knee. Acta Clinica. 2002;12:11-16.
2. Kim HJ, Lee JH, Ahn SE, Park MJ, Lee DH. Influence of Anterior Cruciate Ligament Tear on Thigh Muscle Strength and Hamstring-to-Quadriceps Ratio: A Meta-Analysis. PLoS One. 2016 Jan 8;11(1):e0146234. doi: 10.1371/journal.pone.0146234.
3. Dhillon MS, Bali K, Prabhakar S. Differences among mechanoreceptors in healthy and injured anterior cruciate ligaments and their clinical importance. Muscles Ligaments Tendons J. 2012 Jun 17;2(1):38-43.
4. Hirokawa S, Solomonow M, Luo Z, Lu Y, D'Ambrosia R. Muscular co-contraction and control of knee stability. J Electromyogr Kinesiol. 1991 Sep;1(3):199-208. doi: 10.1016/1050-6411(91)90035-4.
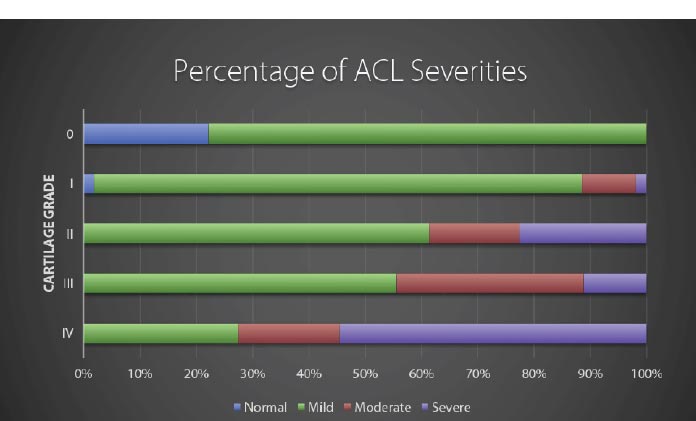
Modified from: Hasegawa A, et al. Anterior cruciate ligament changes in human joint in aging and osteoarthritis. Arthritis Rheum. 2012 Mar; 64(3): 696-704. Figure 4.
6. CURRENT TREATMENT OPTIONS FOR KNEE JOINT CONDITIONS
The standard therapeutic approaches for chronic knee pain include but are not limited to, nonsteroidal anti-inflammatory drugs (NSAIDs), cortisone injections, hyaluronic acid injections, physical therapy, therapeutic exercise, arthroscopic surgery, partial or complete meniscectomy, and total knee replacement. Furthermore, these approaches tend to inhibit soft tissue healing and accelerate the degenerative process rather than stimulate soft tissue repair. Pharmaceutical drugs like NSAIDs decrease these inflammatory substances, but their effect is temporary; they do not address the problem at the source, ligament injury and joint instability. The only restorative treatment to stop the inflammatory substances is to restore normal joint stability.
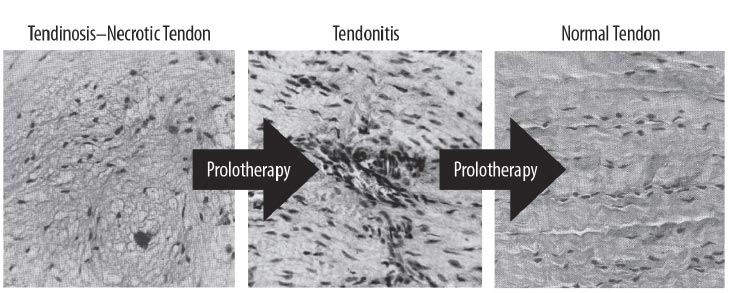
While proper mechanical loading of the knee with exercise can assist muscular imbalance and deficiencies, its effect on the repair of injured ligaments can be ineffective [ 40]. One treatment method that is being used for ligamentous joint instability is prolotherapy [ 41]. The aim of prolotherapy is to stimulate cytokine and growth factor-mediated cell proliferation and matrix deposition, promoting repair over a sustained period (weeks to months) [ 42]. The resulting enlargement and strengthening of damaged ligaments and tendons can reduce joint laxity, improve biomechanics, and decrease pain [ 43]. In a study sample representing 118 knees, patients with unresolved knee pain treated with dextrose prolotherapy showed improvements in many clinically relevant parameters, including exercise ability and overall quality of life [ 44]. The use of many different solutions in prolotherapy has been studied, and their known efficacies are well documented (Table 3).
7. KNEE CONDITIONS ASSOCIATED WITH KNEE INSTABILITY AND THEIR TREATMENT WITH PROLOTHERAPY
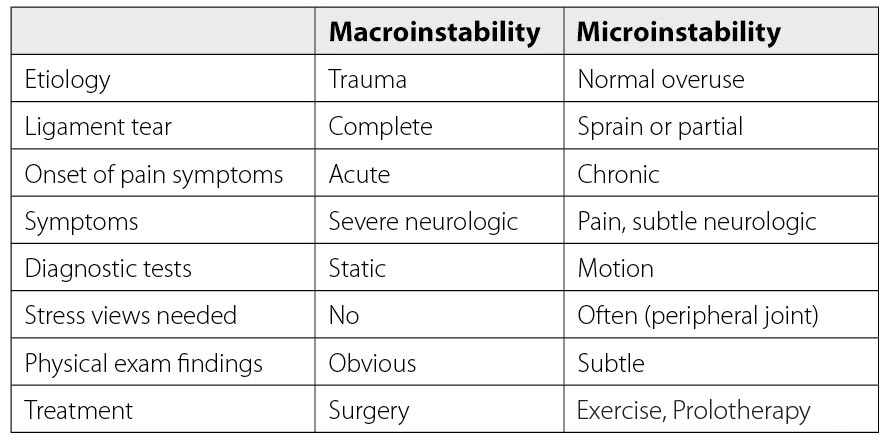
Many knee conditions either result in or occur because of joint instability. Ligament and tendon injuries are major causes of instability that frequently occur in sports and sports-related activities. Macroinstability is often caused by major traumas, is easily diagnosed by physical examination and MRI testing, and is evidenced by significant ligamentous injury (complete tears) and/or joints that dislocate or sublux with minimal force. Microinstability is more prevalent, however, and often comes on insidiously with normal use or minimal injury, persists over a long period of time, and is not easily recognized by physical examination or static radiographic or ultrasound testing. The excessive joint motion of microinstability is present only with more significant force, as occurs with exercise (Fig. 18). While macroinstability typically requires surgery (as in a complete ACL tear), microinstability responds to more conservative measures, such as load management, by reducing compression, exercise therapy, managing comorbidities ( e.g., losing weight), and prolotherapy. Prolotherapy initiates or signals the normally inflammatory healing cascade with the goal of resolving the ligamentous or tendon component of the instability (Fig. 19).
| Solutions | Efficacy |
|---|---|
| Hypertonic dextrose vs. saline | p <0.05 at 12 months [ 1, 2] |
| Hypertonic dextrose vs. PRP* | No difference at 12 months [ 3] |
| PRP vs. hyaluronic acid | p < 0.05 at 12 months [ 4, 5] |
| PRP vs. saline | Non-significant results, no difference [ 6] |
| Bone marrow aspirate vs. PRP and hyaluronic acid | p < 0.0001 at 12 months [ 7] |
| Bone marrow aspirate vs. PRP* | No difference in results at 24 months [ 8] |
1. Rabago D, Patterson JJ, Mundt M, Kijowski R, Grettie J, Segal NA, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013 May-Jun;11(3):229-37. doi: 10.1370/afm.1504. Erratum in: Ann Fam Med. 2013 Sep-Oct;11(5):480.
2. Sit RWS, Wu RWK, Rabago D, Reeves KD, Chan DCC, Yip BHK, et al. Efficacy of Intra-Articular Hypertonic Dextrose (Prolotherapy) for Knee Osteoarthritis: A Randomized Controlled Trial. Ann Fam Med. 2020 May;18(3):235-242. doi: 10.1370/afm.2520.
3. Rahimzadeh P, Imani F, Faiz SHR, Entezary SR, Zamanabadi MN, Alebouyeh MR. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging. 2018 Jan 4;13:73-79. doi: 10.2147/CIA.S147757.
4. Tang JZ, Nie MJ, Zhao JZ, Zhang GC, Zhang Q, Wang B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res. 2020 Sep 11;15(1):403. doi: 10.1186/s13018-020-01919-9.
5. Li S, Xing F, Yan T, Zhang S, Chen F. Multiple Injections of Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Current Evidence in Randomized Controlled Trials. J Pers Med. 2023 Feb 27;13(3):429. doi: 10.3390/jpm13030429.
6. Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA. 2021 Nov 23;326(20):2021-2030. doi: 10.1001/jama.2021.19415.
7. Dulic O, Rasovic P, Lalic I, Kecojevic V, Gavrilovic G, Abazovic D, et al. Bone Marrow Aspirate Concentrate versus Platelet Rich Plasma or Hyaluronic Acid for the Treatment of Knee Osteoarthritis. Medicina (Kaunas). 2021 Nov 2;57(11):1193. doi: 10.3390/medicina57111193.
8. Anz AW, Plummer HA, Cohen A, Everts PA, Andrews JR, Hackel JG. Bone Marrow Aspirate Concentrate Is Equivalent to Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis at 2 Years: A Prospective Randomized Trial. Am J Sports Med. 2022 Mar;50(3):618-629. doi: 10.1177/03635465211072554.
Instability may result as a direct consequence of deficits in the static and dynamic stabilizing forces of injured ligaments and tendons and may also be exacerbated by further soft tissue (ligament) damage secondary to the initial injury. Longer-term sequelae may include other pathological conditions, such as tendinosis or osteoarthritis. Studies of knee injuries and knee biomechanics support multiple possible mechanisms for knee ligament failure, all dependent on the location of the injury [ 45, 46].
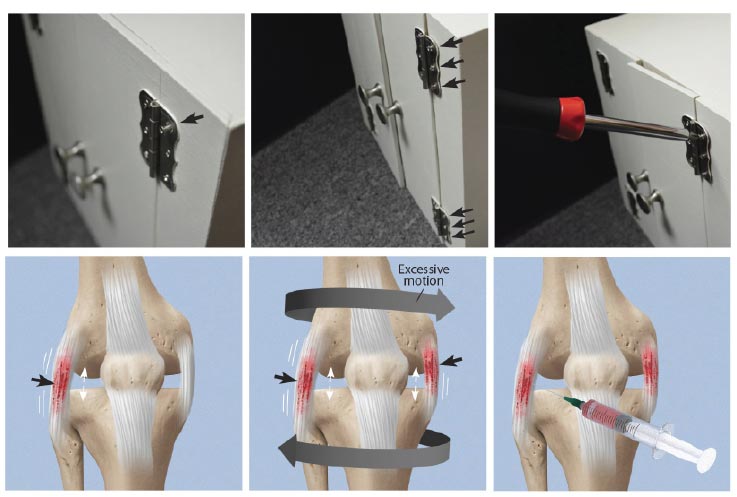
Ligament injury results in abnormal knee kinematics that may cause damage to other tissues in and around the joint, leading to morbidity and pain. A loose screw on a cabinet hinge will cause forces to go to the other screws holding the hinge in place; likewise, once one ligament is injured in the knee, the forces move onto the other stabilizing structures, which can eventually break down. Load on adjacent tendons and ligaments increases in the unstable joint due to greater forces on them as compensatory stabilizing elements, resulting in damage. The use of prolotherapy treatment in such a situation is akin to strengthening all of the loose screws of a hinge with an appropriate screwdriver (Fig. 20).
7.1. Osgood-Schlatter Disease (Knee Pain in Young People)
Osgood-Schlatter disease is one of the most common knee joint conditions of adolescence [ 47, 48] and is believed to result from a traction apophysitis of the tibial tuberosity that occurs due to repeated tensile extension forces from the quadriceps on the weak apophyseal cartilage of the tibial tuberosity. This apophysitis, in turn, results in the avulsion of segments of the anterior cartilage and anterior bone if left untreated [ 49]. While most patients recover with conservative treatment and regain normal patellar length and diameter, some patients at the 2-year follow-up have been shown to continue to suffer from lower endurance, power, and strength compared to healthy controls [ 47].
Knee ligament injury is a lesser-known risk factor for apophyseal and other growth plate injuries [ 50, 51]. Increased external tibial torsion, consistent with the instability of the knee, has also been shown to be a predisposing mechanical factor in the development of Osgood-Schlatter disease [ 52]. Quadriceps muscle tightness, along with increased peak quadriceps torque, is a known compensatory mechanism for joint instability, especially in the ACL-deficient knee [ 53]. ACL laxity causes the quadriceps tendon to become tighter to compensate for an unstable knee, resulting in traction apophysitis of the tibial tuberosity and traumatic avulsion at the open growth plate.
Prolotherapy treatment of Osgood-Schlatter disease is directed at the weakened soft tissue structures, including the patellar tendon and any involved ligaments. In one double-blinded study, young athletes aged 9-17 years with Osgood–Schlatter disease were randomized to dextrose injection, control injection, or a non-injection (supervised exercise) group. Dextrose prolotherapy patients had substantially greater pain reduction during sports activity than either group at follow-up, with many pain-free during sports involvement. At 1 year, 84% of the dextrose-treated knees were pain-free with sports compared to 46% of the lidocaine-treated knees [ 54]. Other clinicians have found dextrose prolotherapy to be safe and effective, aiding in a quicker return to sport for adolescent athletes with recalcitrant Osgood-Schlatter disease [ 55].
7.2. Patellar Pain Syndromes
Patellar pain syndromes are not usually precipitated by trauma but rather occur due to ligamentous injuries, meniscal tears, bony aberrations, muscle imbalances, or a combination of these events. Patellar syndromes, also called patellofemoral pain syndromes, such as patellar subluxation, patellar instability, patellar tendonitis/-osis (jumper’s knee), Osgood-Schlatter disease (tibial apophysitis), and osteochondritis dissecans, can be caused by trauma, but are primarily due to anatomic or biomechanical conditions that are prone to “failure” either in an osseous or soft tissue component. Failing soft tissues, bony aberrations, and lack of sufficient ligament support can produce both static and dynamic instability [ 56]. Patellar instability is often associated with patellofemoral subluxation, misalignments, or dislocation.
Joint instability due to ligament laxity precipitates excessive movement and altered tracking of the patella [ 57]. Physical therapy, patellar taping, orthoses, ice applications, and soft braces are common conservative treatments for patellar pain syndromes [ 58]. They do not address, however, the underlying issues of joint instability secondary to ligamentous injury, tendon injury, or meniscal tears. Prolotherapy can potentially promote nonsurgical repair of the ligament, tendon, and meniscus and has been employed with positive outcomes for patellar pain syndromes associated with joint instability [ 56] (Fig. 21).
7.3. Pes Anserine and Other Tendinopathies
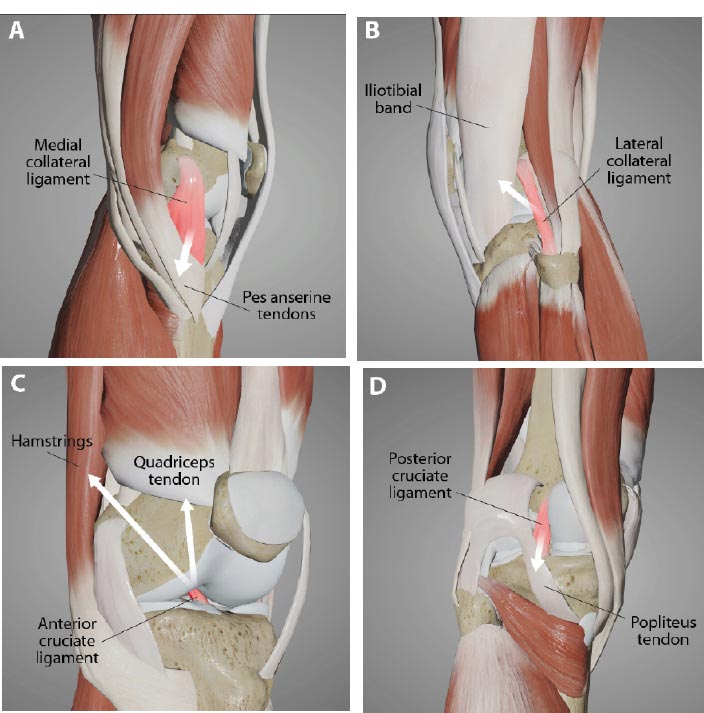
Tendonitis is a frequent diagnosis in sports medicine that has traditionally been viewed as a tendon injury resulting from repetitive mechanical load with a subsequent inflammatory response [ 59]. While some tendon injuries heal well, others linger, potentially because the underlying joint instability causes too much force on them [ 60]. Chronic tendinopathies have been found to be degenerative in nature rather than primarily inflammatory and appropriately termed “tendinosis,” reflecting a focus on damage rather than inflammation. Tendinopathy is remarkable for mucoid degeneration and a loss of collagen continuity, which requires appropriate treatment and management regimens consistent with the degenerative pathology [ 61, 62]. Through the ligamento-muscular reflex, muscles, and thus their corresponding tendons around the knee, are recruited for stability. The most common ones are the hamstrings and quadriceps for anterior knee instability, pes anserine for medial instability, iliotibial band for posterolateral instability, and popliteus when posterior knee instability is present (Fig. 22).
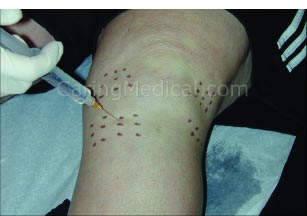
Injecting the ligament attachment sites is the most important part of comprehensive Prolotherapy.
Pes anserine tendonitis is one of the most frequent types of chronic knee injuries [ 63]. The pes anserine tendon group prevents the lower leg from twisting outward while running. The tendinosis of this structure is characterized by inflammation of the medial knee and often coexists with other knee disorders [ 64]. During walking, pressure from the weight of the body is medial to the knee rather than lateral, applying a varus torque at the knee, increasing the compressive force and subsequent breakdown at the medial aspect [ 65]. Chronic stress from activity or contusion to the pes anserine bursa near the tibial insertion may precipitate the inflammation. An underlying or coexisting medial collateral ligament injury causing joint instability would put additional stressors on the pes anserine tendons as they try to stabilize the medial joint line. Prolotherapy can be used in the medial knee joint line, including the pes anserine area, to relieve pain and stabilize the knee [ 66].
7.4. Chondral Defects and Osteoarthritis
Osteoarthritis is characterized by a breakdown of articular cartilage. Articular cartilage is comprised of connective tissue nourished by the surrounding joint fluid. It lacks blood vessels, lymphatics, and nerves and has a limited ability for healing and repair. As such, the preservation of healthy articular cartilage is essential for joint health, and the health of the cartilage is dependent on the normal loading process that occurs through the usage of the joint [ 66, 67]. Articular cartilage has a great ability to withstand high cyclic loads [ 68]; excessive loading, rotational forces, and trauma, however, can cause cell death and result in damage to the extracellular matrix and cartilage. When damaged, the unique and complex structure of articular cartilage makes treatment and repair a significant challenge, especially considering that injury to articular cartilage is recognized as a cause of significant musculoskeletal morbidity [ 69].
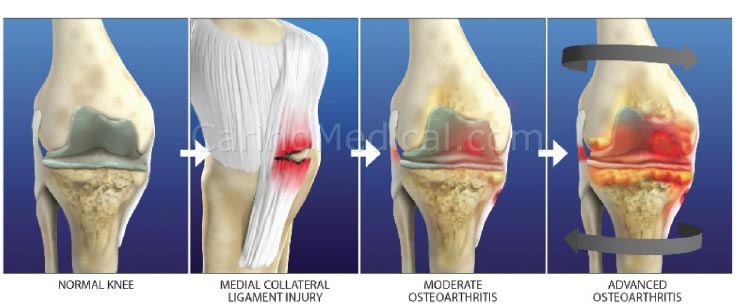
The progression of chondral lesions ultimately results in osteoarthritis, a chronic degenerative joint disorder characterized by articular cartilage destruction and osteophyte formation. Although loss of articular cartilage plays a pivotal role in osteoarthritis, the condition is often preceded by joint instability due to soft tissue lesions, such as ligament injury (Fig. 23). Injured or overstretched ligaments alter joint biomechanics, and the resultant joint instability allows for an uneven gliding motion and increased pressure on the articulating bones, eventually leading to osteoarthritis [ 64] (Fig. 24). Studies in mouse models of osteoarthritis indicate that the progression of the disorder is related to the severity of joint instability [ 70, 71]. Subsequent injury to the knee is also one of the strongest predictors of the development of OA in that joint [ 72, 73]. In a Johns Hopkins University prospective cohort study on young adults with knee trauma, 13.9% developed radiographic and symptomatic osteoarthritis over a median follow-up of 36 years, compared to 6.0% of those without knee injury [ 74]. Notably, slightly more than half (57%) of people who have a traumatic knee injury will develop knee OA in their lifetime [ibid].
Prolotherapy studies with dextrose, platelet-rich plasma, and bone marrow have reported success with chondromalacia patella and osteoarthritis [ 75, 76] (Fig. 25). Randomized controlled studies have shown the effectiveness of dextrose prolotherapy in treating osteoarthritic knees. Of these 3 studies, those of Rabago et al. and Reeves and Hassanein were double-blinded, the latter study showing efficacy in patients with and without ACL laxity [ 77, 78]. The third study, conducted by Dumais et al., was a randomized crossover study in which prolotherapy and exercise therapy were combined in alternating sequences [ 79]. All 3 studies showed a significant gain from prolotherapy treatment with respect to both pain and functional capacity. Additionally, in a study on knee and hip osteoarthritis, Hauser and Woldin reported the results of a combined dextrose prolotherapy and bone marrow prolotherapy treatment regimen in patients with radiographic osteoarthritis and observed pain relief, improved joint function, and enhanced quality of life [ 80]. Prolotherapy injections target the multiple potential pain generators inside and around the knee joint [ 81] (Fig. 26).
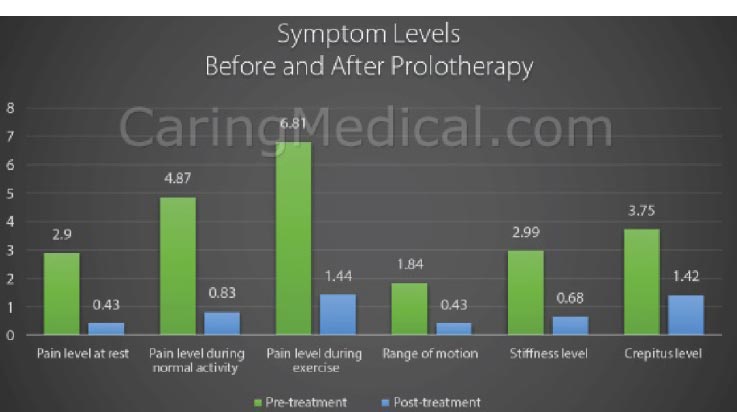
Modified from: Havil S. Maddela, BS, MPH, CPH & Ross Hauser, MD. The Use of Prolotherapy for Chondromalacia Patella (Patellofemoral Pain Syndrome). Journal of Prolotherapy. 2018;10:e1000-e1008.
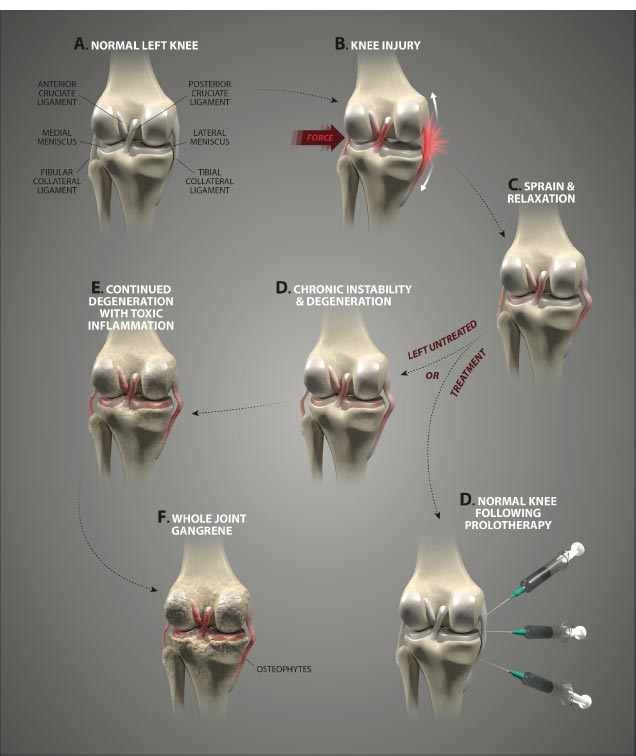
CONCLUSION
Knee pain is a common malady, with ligamentous knee joint instability constituting a causative or associative factor in multiple painful knee joint conditions. Ligamentous knee instability causes destructive joint forces to occur. The interplay between the various structures of the knee creates a dynamic whereby injury to one area generates increased forces on another, or injury or weakness in one soft tissue structure can cause wear and tear, propagating further instability and degeneration of another structure. A strong relationship exists between knee joint instability and the development of other disabling knee conditions, including anterior and posterior cruciate ligament and medial collateral ligament injuries, posterolateral and meniscal injuries, patellar pain syndromes, tendonitis/-osis, and chondral defects, along with a higher risk of developing osteoarthritis of the knee.
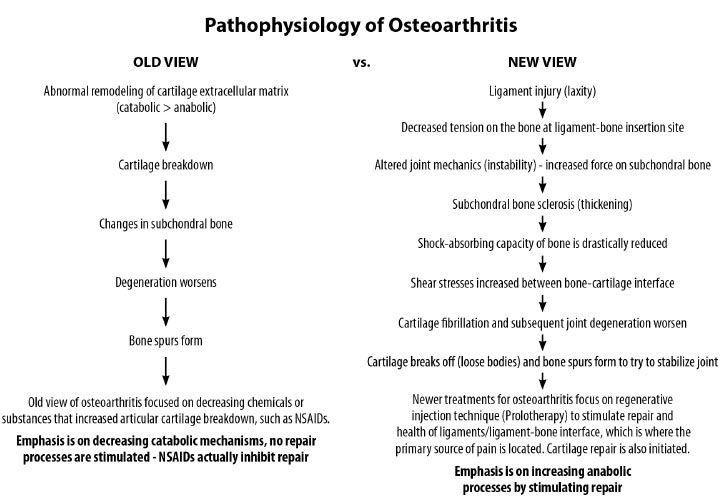
Ligaments are key structures in the stability of the knee joint, yet due to their especially high density of innervation close to the bony ligament insertion sites and persistent deficits in healing long after injury, ligament injury is a major cause of pain and joint instability that is associated with several chronic knee conditions. Joint instability causes the force per unit area on knee structures, including the articular cartilage, to increase substantially, contributing to its breakdown. Joint instability can be demonstrated by functional clinical exams (with or without ultrasound verification of joint opening) and through such signs as crepitus, grinding, and tenderness. In osteoarthritis, the catabolic processes are stronger than the reparative anabolic processes. Treatments to decrease the catabolic process or joint destruction (especially in a degenerated joint) fall short since repair is not being accomplished. Regenerative treatments are needed to stimulate anabolic repair.
Regenerative injection therapy (Prolotherapy) is a treatment designed to stimulate the repair of the injured ligaments and other structures to enhance joint stability by tightening and strengthening ligaments and tendons. Prolotherapy has been shown to be efficacious in reducing the pain and disability of many chronic painful knee conditions, including Osgood-Schlatter disease, patellofemoral pain syndrome, patellar tendinopathy, and osteoarthritis.
LIST OF ABBREVIATIONS
| OA | = Osteoarthriti |
| HCUP | = Healthcare Cost and Utilization Project |
| AHRQ | = Healthcare Research and Quality |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.