Anesthetic Effects of Sevoflurane on the Mouse Somatosensory Cortex: A Flavoprotein Fluorescence Imaging Study
Abstract
Objective:
Sevoflurane, a volatile inhaled anesthetic, is used clinically for general anesthesia in humans. However, the mechanism of action of sevoflurane is not fully understood. We used transcranial flavoprotein fluorescence imaging to visualize somatic sensory cortex responses to noxious stimuli in mice without and with sevoflurane inhalation anesthesia at different concentrations to investigate sevoflurane effects in mice.
Methods:
A bipolar stimulating electrode was inserted into the left buccal region of the mouse, and changes in flavoprotein fluorescence intensity in the right somatic sensory cortex were recorded before and after electrical stimulation. Measurements were taken while the mouse was awake, at four levels of sevoflurane concentration (0.5%, 1.0%, 1.5%, and 2.0%; 5 min each), and at 10, 20, and 30 min after the end of sevoflurane inhalation.
Results:
During the awake period, flavoprotein fluorescence intensities in the right sensory cortex decreased after the onset of electrical stimulation, but after 0.9 s, the fluorescence intensity began to increase, reaching a peak value at 2.1 s. This biphasic response significantly decreased at 0.5% sevoflurane and completely disappeared at sevoflurane concentrations above 1.5%, and restored 10 min after cessation of the sevoflurane inhalation. Furthermore, low concentrations of sevoflurane had little effect on the reduction of receptive fields or the conduction of excitation.
Conclusion:
We conclude that low concentrations of sevoflurane have little effect on the reduction of receptive fields or the conduction of excitation, and that sevoflurane concentrations above 1.5% completely abolish the sensory cortex response elicited by noxious stimulation.
1. INTRODUCTION
The somatosensory cortex (SC) is the region of the cerebral cortex that processes sensations, such as pain [1]. For physiological studies of brain function in laboratory animals, electrophysiological techniques have been widely used; however, recent technological advances have led to the use of optical imaging methods that enable spatiotemporal recordings[2-6]. Some optical imaging methods use voltage-sensitive dyes, but these approaches are invasive and require the brain to be exposed and stained. By contrast, flavoprotein fluorescence imaging (FFI) does not require exposure of the brain and allows transcranial measurement of autofluorescence. Flavoprotein, one of the intracellular mitochondrial electron transport proteins, changes from reduced flavin mononucleotide (FMNH2) to oxidized flavin (FMN) when oxygen metabolism is enhanced by increased intracellular calcium concentration due to neuronal activity in the brain. Oxidized flavin emits green autofluorescence when exposed to blue excitation light [7]. Methods using this endogenous signal to identify excitatory areas in the SC that react to stimulation of specific sites have been developed and applied to higher brain research [8-18]. In most of these studies, urethane or ketamine-xylazine mixtures have been used as anesthetic agents.
Sevoflurane (sevo), a volatile inhaled anesthetic, is clinically used for general anesthesia in humans. Sevo has a minimum alveolar concentration of approximately 1.7%, a blood-gas partition coefficient of 0.63, moderate anesthetic potency, rapid induction of arousal, and low airway irritation [19-21]. The sedative and analgesic mechanism of sevo is not fully understood. Several explanations have been proposed focusing on gamma-aminobutyric acid (GABA)A receptors [22-29]. In a study examining the effects of sevo on cortical activity in experimental animals, spontaneous activity detected by electroencephalography was suppressed in a concentration-dependent manner and disappeared at high concentrations of inhaled anesthetics, but evoked potentials to visual stimuli remained [30]. Using FFI, cortical responses to tactile, nociceptive, visual, and acoustic stimuli under urethane anesthesia have been reported, but such responses have not been reported for sevo anesthesia [8-18].
In this study, we visualized SC responses to electrical stimulation in the subcutaneous buccal region of mice using FFI and investigated the effects of sevo anesthesia.
2. MATERIALS AND METHODS
2.1. Animals
All animal experiments were approved by the Laboratory Animal Care and Use Committee of Uekusa Gakuen University (approval number: URAC19-06) and complied with the Guidelines for Care and Use of Laboratory Animals released by the National Research Council of the National Academies (8th edition, revised 2011) and with the Guiding Principles for Care and Use of Animals of the Physiological Society of Japan. We used 11 adult male ICR mice (Sankyo Lab Service, Tokyo, Japan) aged 8-12 weeks.
Surgical procedures were carried out under sevo anesthesia with continuous inhalation at 3.0%, followed by subcutaneous injection of lidocaine hydrochloride jelly (2.0%). The skull was exposed, and liquid paraffin was applied to prevent the drying of the skull surface. A chamber frame was bonded, fixed to the mouse skull with dental resin cement (Sun Medical CO, Shiga, Japan), and attached to a head fixation device (MAG-2, Narishige, Tokyo, Japan). A bipolar electrode was inserted under the skin of the left buccal region of the mouse. After confirming that the mouse was awake by interrupting sevo anesthesia, changes in flavoprotein fluorescence induced by electrical stimulation of the left buccal subcutaneous region were recorded using a MiCAM02 camera (Brainvision, Tokyo, Japan) (Fig. 1). Electrical stimulation was performed for 0.5 s with 1 ms duration, 1 V intensity, and 20 Hz frequency using an electrical stimulator (SEZ3100, Nihon Kohden, Tokyo, Japan). The body temperature of the mice was maintained at 37°C using a thermal pad, and the experiment was performed with spontaneously breathing mice.

2.2. Flavoprotein Fluorescence Imaging
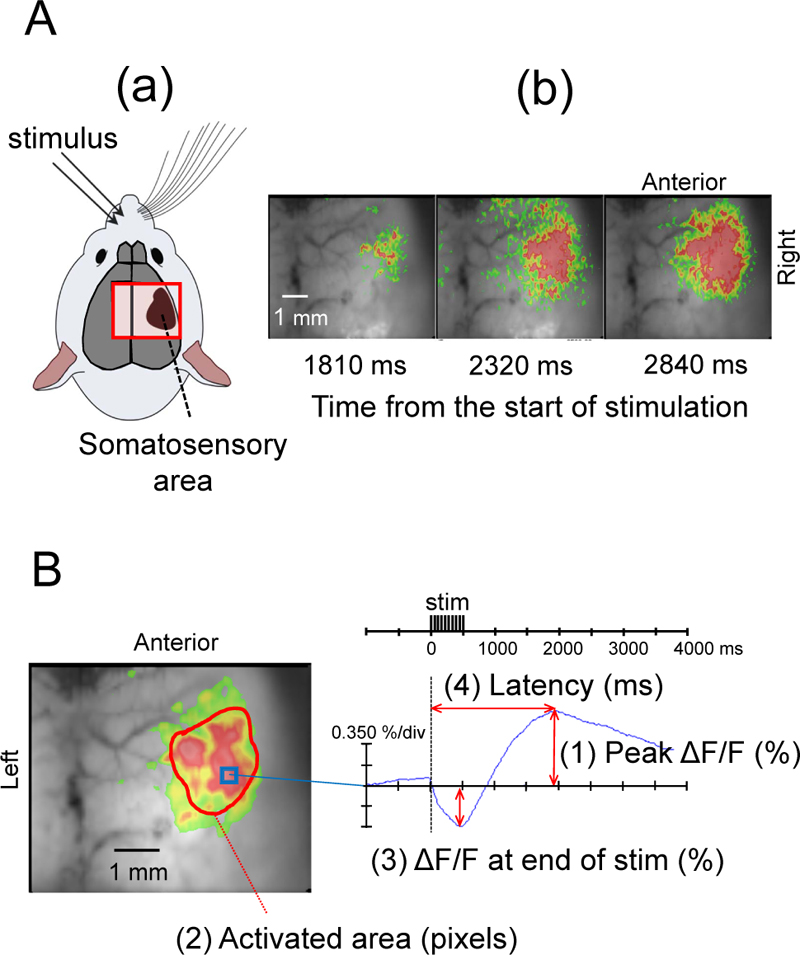
We performed the same FFI experiment previously reported by Shibuki et al. [9], and confirmed the occurrence of fluorescence changes in the right SC following stimulation of the left buccal region of mice (Figs. 1 and 2A). When the SC was exposed to blue light (450-490 nm) from a high-intensity LED light source (LEX2-B, Brainvision), the cortical tissues emitted green autofluorescence (500-550 nm), which was recorded by a cooled CCD camera system (MiCAM02) using an upright fluorescence microscope (THT, Brainvision) with an objective (magnification: 1.0) (Fig. 1).
Optical imaging and data analysis were performed using the MiCAM02 hardware and software package (BV Ana: Brainvision). The camera captured images of 96 × 64 pixels (6 mm × 5 mm). Total frame acquisition was set to 512. The sampling time was 10 ms/frame; therefore, the total recording time was 5120 ms. The acquisition was triggered by electrical stimuli. The trigger signal was activated after one-quarter of the total recording time, corresponding to 1280 ms after starting acquisition. To improve the signal-to-noise ratio, we averaged signals detected in 10 consecutive trials at 0.15 Hz. Data were displayed using a 3 × 3 pixels spatial filter and a 25% dF/Fmax filter (Fig. 2A, B). Measurements were taken while the mouse was awake, at four levels of sevo concentrations (0.5%, 1.0%, 1.5%, and 2.0%; 5 min each), and at 10, 20, and 30 min after the end of sevo inhalation. To compare the effects of different sevo concentrations, we measured the maximum flavoprotein fluorescence intensity (peak ΔF/F), activated area at the time of maximum fluorescence increase (activated area), the difference in fluorescence decrease at the end of electrical stimulation (ΔF/F at the end of stim), and time from the start of stimulation to the point of maximum flavoprotein fluorescence (latency) (Fig. 2).
3. RESULTS
Electrical stimulation of the left buccal subcutaneous region of mice elicited a biphasic change in flavoprotein fluorescence intensity in the right SC barrel field with a decrease immediately after the stimulation, followed by a slow increase (Fig. 2). A typical example during the awake period is shown in Fig. (2). After electrical stimulation, flavoprotein fluorescence intensity changed in the SC barrel field and then spread throughout the SC (Fig. 2A).
Fig. (2B) shows that the flavoprotein fluorescence intensity in the right SC decreased after the onset of electrical stimulation, but after 0.9 s, the fluorescence intensity began to increase, reaching a maximum value at 2.1 s.
At the sevo 0.5% level, the biphasic change in flavoprotein fluorescence intensity in the SC was decreased compared to the awake state; at sevo 1.5% and sevo 2.0%, the observed changes in fluorescence intensity in the SC disappeared; at 10 min after cessation of sevo inhalation, the biphasic signal returned; and 20 and 30 min after stopping the anesthetic drug, the change in flavoprotein fluorescence response was restored (Fig. 3).
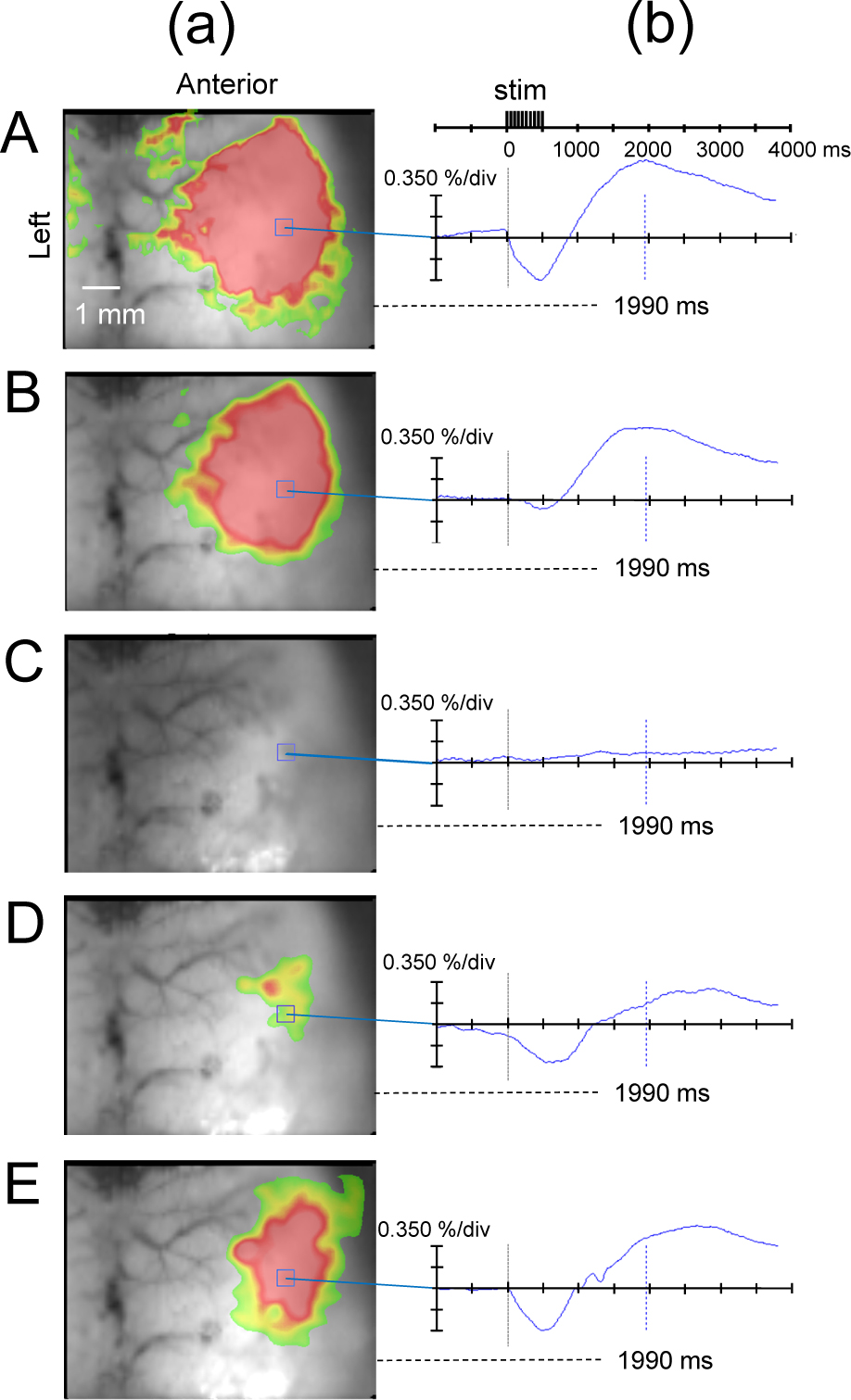
(a) Flavoprotein fluorescence imaging at 1990 ms from the start of stimulation and (b) temporal change in ΔF/F at each sevoflurane concentration. A: Awake state. B: Inhalation of sevoflurane 0.5%. (c): Inhalation of sevoflurane 1.5%. (d): 10 min after cessation of sevoflurane inhalation. (e): 20 min after cessation of sevoflurane inhalation. The biphasic change in flavoprotein fluorescence intensity in the SC decreased at the sevoflurane 0.5% level, compared to the awake state, disappeared at the sevoflurane 1.5% level, and restored at 10 min after cessation of sevoflurane inhalation. stim, stimulus.
Peak ΔF/F was 0.79 ± 0.59% in mice that were awake and it decreased to 0.45 ± 0.49% and 0.19 ± 0.30% at sevo 0.5% and sevo 1.0% levels, respectively. No stimulation-induced changes in flavoprotein fluorescence intensity were observed for sevo concentrations of 1.5% and 2.0%. After cessation of the sevo inhalation, peak ΔF/F increased to 0.47 ± 0.09% after 10 min, 0.60 ± 0.32% after 20 min, and 0.70 ± 0.39% after 30 min. Peak ΔF/F values significantly differed between the awake state and sevo 1.0% level (Fig. 4A).

The activated area tended to decrease from the time the animal was awake to the time of sevo 0.5% and 1.0%, but this value increased from 10 min to 20 min and 30 min after sevo cessation. Significant differences were found between the awake state and sevo 1.5% concentration, as well as between the awake state and sevo 2.5% level (Fig. 4B).
The values for ΔF/F at the end of stim were -0.66 ± 0.58% at the awake state, -0.28 ± 0.21% at sevo 0.5%, -0.26 ± 0.27% at sevo 1.0%, -0.56 ± 0.18% at 10 min off, -0.50 ± 0.13% at 20 min off, and -1.11 ± 1.30% at 30 min off. The values in the awake state were significantly different from those at sevo 1.5% and sevo 2.0% levels (Fig. 4C).
The latency was 2151 ± 775.9 ms at the awake state, 1679 ± 709.2 ms at sevo 0.5%, 2433 ± 782.4 ms at sevo 1.0%, 2259 ± 922.7 s at 10 min off, 2518 ± 984.8 ms at 20 min off, and 1597 ± 677.1 ms at 30 min off. No significant differences were found for the parameter latency (Fig. 4D).
4. DISCUSSION
In this study, we measured biphasic changes in flavoprotein fluorescence intensity, which is thought to be correlated with pain perception in the SC barrel field induced by electrical stimulation of the buccal subcutaneous region of mice. With increasing sevo concentrations, the peak value of the stimulus-induced flavoprotein fluorescence change decreased at sevo 0.5% to about 57% and at sevo 1.0% to about 24% of the value in awake mice. Following discontinuation of sevo administration, this parameter was restored to about 60% after 10 min and nearly fully recovered after 30 min. During the awake period, the flavoprotein fluorescence intensity in the right SC decreased after the onset of electrical stimulation, and after 0.9 s, the fluorescence intensity began to increase, reaching its maximum at 2.1 s.
Aerobic energy metabolism is important for brain activity, and mitochondria are abundant in brain tissue. The activation of aerobic energy metabolism and the resulting oxidation of mitochondrial flavoproteins are mechanisms underlying the observed autofluorescence responses. These well-known facts suggest that FFI faithfully reflects neural activity in vivo [8]. In mice, FFI can be useful because it allows transcranial imaging, causes less surgical damage, and is more likely to produce stable recordings [11]. However, fluorescence responses of flavoproteins have a time lag because various metabolic reactions are involved in changes in flavoprotein fluorescence. The initial response within 0.5-0.8 s after the onset of stimulation is not affected by blood vessels, but the latter half of the fluorescence response is influenced by hemodynamic changes [9, 31]. In the present study, we continuously took recordings from the awake state over anesthesia with various sevo concentrations to the recovery period; therefore, the recordings may have been affected by oxygen consumption and hemodynamics due to neural activity.
The biphasic response suggested the percentage of oxidized flavoproteins to be decreased during or immediately after stimulation and increased thereafter. Chisholm et al. reported that hypoxia induced a characteristic change in the cortex with the preservation of oxidized flavoprotein in the periarterial tissue and reduced flavoproteins in distal tissues and near veins [32]. Shibuki et al. conducted experiments using FFI to identify the sites in the SC that respond to tactile, visual, and auditory stimuli in rodents, and reported temporospatial recordings of regions with excitation in the SC [9]. Furthermore, they showed the activation of aerobic energy metabolism and the resulting oxidation of mitochondrial flavoproteins to be the mechanisms underlying the autofluorescence responses. In in vivo experiments, the fluorescent responses were emphasized by the hemodynamic responses [8]. Decreases in flavoprotein fluorescence intensity after stimulation have been reported in certain layers and under anoxic conditions [32-34]. Tsytsarev et al. tested in vivo the cell-penetrating phosphorescent oxygen-sensitive probe NanO2-IR and reported that whisker stimulation led to a decrease in O2, followed by an increase in O2 in the SC [35]. The findings of the present study suggest that the percentage of reduced flavin increases during and immediately after stimulation because O2 is utilized as neurons become active, followed by an increase in the percentage of oxidized flavin due to increased blood flow and other factors.
Urethane or ketamine-xylazine have been used as anesthetics in studies examining cortical responses to sensory stimuli in laboratory animals. Under these anesthetics, cortical activity responses elicited by various sensory stimuli remain [2-6, 8-18]. In the present study, the changes in flavoprotein fluorescence in the cortex induced by stimulation of the buccal area of mice decreased by a sevo concentration of 1.0% and completely disappeared at concentrations of 1.5% or higher, but they restored at 10 min after stopping sevo administration. Arena et al. reported that sevo reduced cortical spontaneous activity in a dose-dependent manner and the dose-dependent potentiation induced by sevo gradually converted into a dose-dependent depression with increased stimulation frequency [30]. This suggests that an increase in external stimuli not only abolishes the capacity-dependent facilitation of sensory responses but also underlies the capacity-dependent inhibition by sevo. In our study, the decrease in active area and prolongation of latency in response to stimuli seen with sevo 1.0% inhalation was aggravated by sevo 1.5% and sevo 2.0% administration, leading to the complete abolishment of signal changes. This suggests that low sevo concentrations have little effect on the reduction of the stimulus-responsive SC receptive field or on the conduction of excitation; however, when a certain concentration is reached, a conduction block is presumed to occur.
Although many of the mechanisms of action of anesthetics remain unclear, GABAA receptors are involved in the pharmacological effects of many anesthetics. Sevo and other volatile inhaled anesthetics act on inhibitory GABAA receptors and directly inhibit glutamate receptors [24-27]. GABAA receptors are the most abundant inhibitory neurotransmitter receptors in the brain. Drexler et al. reported that sevo alters cortical down-states and that administration of the GABAA receptor antagonist bicuculline attenuates the effects of sevo in in vitro experiments, suggesting that sevo enhances GABAA receptor-mediated inhibition at low concentrations but maintains cortical activity [36]. This supports our proposed explanation of the observed sevo effects.
In this study, we did not perform additional pharmacological experiments, including the determination of the long-lasting effect of sevo; however, pharmacological experiments using FFI may illuminate the mechanisms underlying sevo effects in vivo. The use of calcium imaging, which has a higher time resolution than FFI [17], in combination with FFI, may also help to elucidate the mechanism of action of anesthetics.
CONCLUSION
In summary, we used FFI to transcranially visualize SC responses to painful stimuli in mice without and with sevo inhalation anesthesia at four different concentrations. We found that, unlike urethane or ketamine-xylazine, sevo completely abolishes the SC response elicited by noxious stimulation. Our results suggest that sevo blocks the conduction of sensory information. Since the mechanism of action differs depending on the type of anesthesia, it will be necessary to continue to study the mechanism of action of various anesthetics from various angles using FFI.
LIST OF ABBREVIATIONS
| SC | = Somatosensory cortex |
| GABA | = Gamma-aminobutyric acid |
AUTHORS’ CONTRIBUTIONS
All authors contributed to the writing, editing, and final revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animal experiments were approved by the Laboratory Animal Care and Use Committee of Uekusa Gakuen University (approval number: URAC19-06).
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. All animal research procedures were followed in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals (published by the National Academy of Sciences, The National Academies Press, Washington, D.C.).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.